Jennifer M. Taylor, MD presented “Bladder Cancer and Immunotherapy” during the 23rd Annual Innovations in Urologic Practice on September 14, 2018 in Santa Fe, New Mexico.
How to cite: Taylor, Jennifer M. “Bladder Cancer and Immunotherapy” September 14, 2018. Accessed [date today]. https://dev.grandroundsinurology.com/bladder-cancer-and-immunotherapy/
Bladder Cancer and Immunotherapy – Summary:
Jennifer M. Taylor, MD, MPH, provides a brief overview of the basic scientific concepts of cancer cell suppression with checkpoint inhibitors. She also reviews the initial clinical trials for atezolizumab and pembrolizumab, larger trials for various immunotherapies for bladder cancer, toxicity reports, and ongoing research regarding these drugs in different combinations and disease states.
Abstract:
Advanced urothelial carcinoma (UC) is the ninth most common cancer worldwide, as well as the eighth most common cause of cancer death among men in the United States. Unfortunately, the prognosis of UC with first-line combination chemotherapy with platinum is 14-15 months. Additionally, the prognosis after failure of platinum combination is 4-7 months. Checkpoint inhibitors and immunotherapy represent an opportunity for improved survival for these patients.
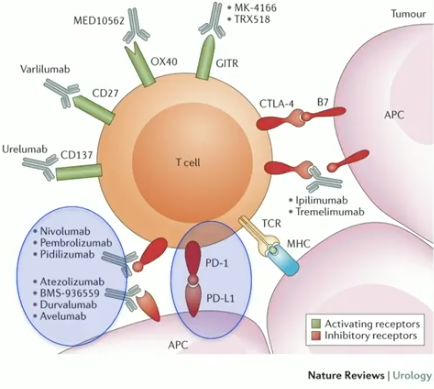
PD-1/PD-L1 pathway checkpoint inhibitors suppress cancer cell proliferation through interactions between T-cells and tumor cells. Data shows that this blockade of the PD-1/PD-L1 pathway improves overall survival in multiple solid tumors, including bladder cancer. This presentation reviews the first atezolizumab and pembrolizumab trials in advanced UC, which overall showed responses in a heavily pretreated populations with advanced disease. The discussion also covers larger trials for checkpoint inhibitors in UC, most of which are investigating these drugs as second-line therapies. However, the IMVigor 210 (Cohort 1) and Keynote-052 trials are considering atezolizumab and pembrolizumab as first-line therapies for cisplatin-ineligible patients.
With the emergence of these immunotherapies, clinicians must become familiar with immune-related toxicities, such as pneumonitis, transaminitis, and rashes. Compared to chemotherapy, however, these drugs are much better tolerated. The FDA has currently approved five checkpoint inhibitors for second-line treatment of metastatic UC. Ongoing trials are are also investigating checkpoint inhibitors in other patient statuses, including muscle and non-muscle invasive bladder cancer (NMIBC). Further research in bladder cancer immunotherapy is focusing on checkpoint inhibitors in combination with chemotherapy as a second-line option, as well as in combination with targeted therapy, as neoadjuvant treatment, and for BCG unresponsive NMIBC patients unfit or unwilling to undergo radical cystectomy.
About Innovations in Urologic Practice
Innovations in Urologic Practice (IUP) is an annual CME-accredited conference devoted to updating urologists on the rapidly changing healthcare environment. Topics focus on innovative diagnostic and treatment strategies, controversies, new and currently developing technologies, and challenges in today’s urologic practice. Dr. Taylor presented this lecture during the 23rd IUP in 2018. Please visit this page in order to learn more about future IUP meetings.