Erik S. Mittra, MD, PhD, presented “Peptide Receptor Radionuclide Therapy for NETS” during a joint session with SNMMI at the 2018 ASCO Annual Meeting.
How to cite: Mittra, Erik “Peptide Receptor Radionuclide Therapy for NETS” June 2, 2018. Accessed [date today]. https://dev.grandroundsinurology.com/peptide-receptor-radionuclide-therapy-for-nets/
Peptide Receptor Radionuclide Therapy for NETS – Summary:
Erik S. Mittra, MD, PhD, explains the literature and rationale behind peptide receptor radionuclide therapy (PRRT) for neuroendocrine tumors (NETs), including reviewing the NETTER-1 trial that led to its approval. He then discusses the practical aspects and clinical practice of PRRT, how to integrate it in a hospital setting, and future directions for this therapy.
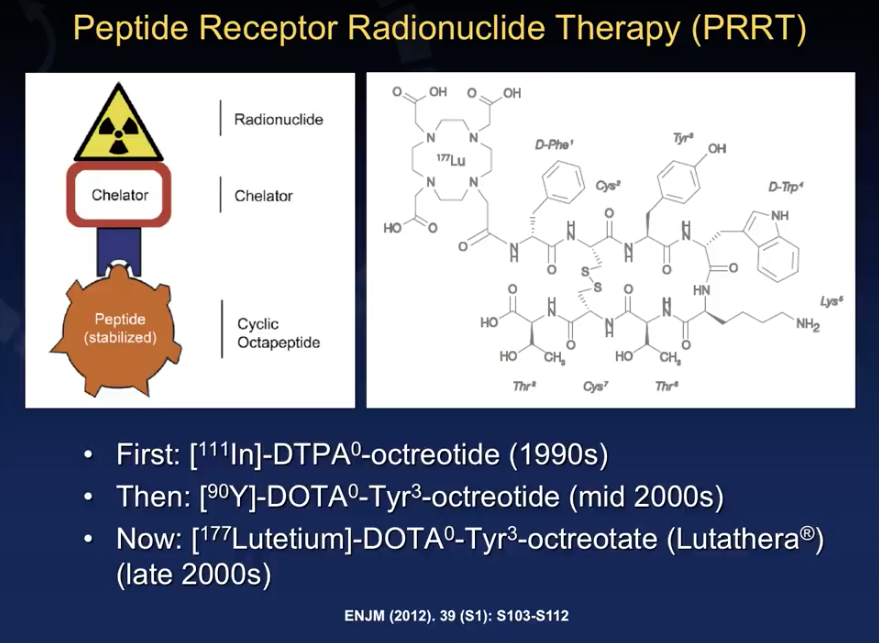
Lutetium Mechanisms of Action for NETs
Since the 1990s, physicians have used the molecules [111in]-DTPA0-octreotide (octreotide) and [90Y]-DOTA0-Tyr3-octreotide (yttrium) in PRRT for NETs. However, [177Lutetium]-DOTA0-Tyr3-octreotide (lutetium, brand name Lutathera) received FDA approval of January of this year.
Lutathera is an agonist for somatostatin (SST), a receptor overexpressed on the surface of NET cells. After concentrating into the NET site, Lutathera binds to somatostatin receptors. Then, the molecule internalizes in the cells and delivers radiation inside the cells, inducing tumor cell death. Unfortunately, like in any radioisotope therapy, the radiation dose may extend beyond the cell and cause toxicity.
NETTER-1
NETTER-1, a randomized, prospective, multicenter, phase III trial, published in The New England Journal of Medicine in 2017, led to the FDA approval of Lutathera. Although more European centers than American centers participated in the study, the American centers enrolled more patients. PRRT had been available outside of trials for many years in Europe. But, American patients did not have access to the therapy outside trials at the time.
The study tested a Lutathera arm against an octreotide LAR arm, with a primary objective of progression-free survival (PFS). Secondary objectives included objective response rate (ORR), overall survival (OS), time to progression, safety and tolerability, and quality of life (QOL).
Eligibility criteria for NETTER-1 required that adult patients had a metastatic or locally advanced NET with a Ki67 index less than 20%. Patients must have been progressing while on a standard dose of octreotide, had somatostatin receptor-positive disease, and had a good kidney function status.
The results of NETTER-1 were dramatically positive. It concluded that Lutathera was superior to octreotide in terms of PFS and ORR. The current findings suggest an increased OS but require further confirmation. Additionally, results were favorable to Lutathera in terms of toxicity and QOL outcomes.
Practical Aspects
While Lutathera seems like a wonderful therapy based on NETTER-1 results, it may not be appropriate for all patients. Dr. Mittra stresses the importance of treating the aforementioned NETTER-1 eligibility criteria as parameters for patient selection when utilizing Lutathera in clinical practice.
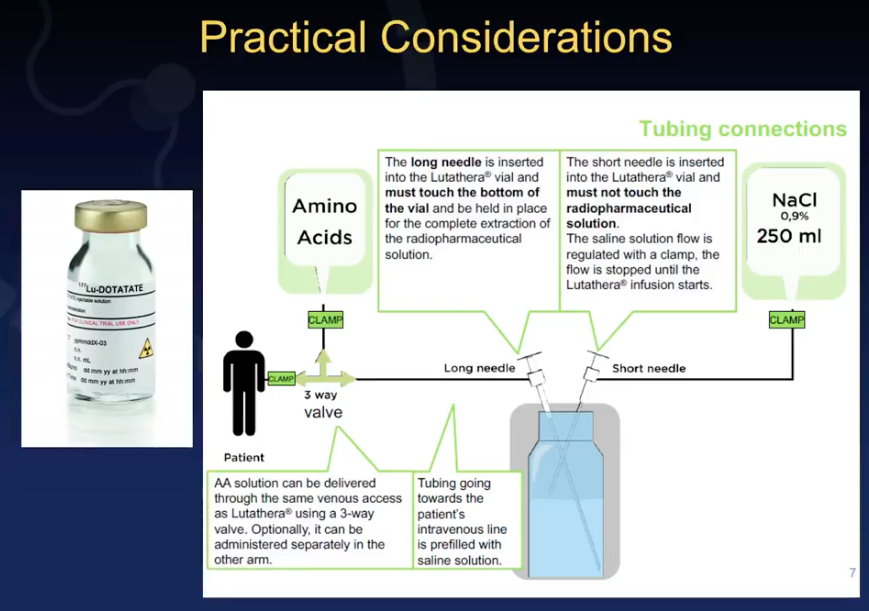
There are also practical challenges clinicians face in terms of physically delivering Lutathera doses to patients. Dr. Mittra reviews guideline recommendations for a two-needle approach, or the gravity method, as well as an automated syringe pump method. Furthermore, he discusses considerations regarding timing of administration, infusion space inside an institution, and precautions when releasing a patient.
Current Issues and Opportunities for Improvements in PRRT
Dr. Mittra highlights the constraints of Lutathera in clinical practice. These limitations include limited amount of academic centers available to perform this therapy and available infusion space. Gaining access to the amino acids necessary for administration can be a challenge, as well. Due to the therapy’s newly-approved status, patients often experience insurance authorization and reimbursement delays.
This new method calls into question the role that nuclear medicine physicians and oncologists play in evaluating patients for this therapy, treating patients, and conducting follow-up. Also, the lack of centralized guidelines in PRRT for NETs is cause for concern.
Future Directions
Dr. Mittra argues that future research into PRRT should focus on sequencing and possible combinations. Additionally, research should explore the possibility of salvage PRRT, intra-arterial administration, novel peptides with higher binding affinity to the SST receptor, the role of yttrium in patients with high-volume tumors, as well as improved dosimetry, radioprotection, and response assessment.