Juanita M. Crook, MD, FRCPC, presented “Does Radiation Therapy Favor Radical Prostatectomy in Long-Term QOL?” during the Southwest Prostate Cancer Symposium on April 15, 2018 in Scottsdale, Arizona.
How to cite: Crook, Juanita M. “Does Radiation Therapy Favor Radical Prostatectomy in Long-Term QOL?” April 15, 2018. Accessed [date today]. https://dev.grandroundsinurology.com/does-radiation-therapy-favor-radical-prostatectomy-in-long-term-qol/
Does Radiation Therapy Favor Radical Prostatectomy in Long-Term QOL? – Summary
Juanita M. Crook, MD, FRCPC, discusses the challenges of assessing modalities for the management of high-risk prostate cancer in terms of quality of life (QOL) outcomes. She then reviews QOL data from the ProtecT trial, the efficacy and toxicity of presented for ASCENDE-RT trial, and data from two large, mature prospective databases. She also shares her opinion on the “tri-modality” approach.
Background Issues
While physicians are increasingly managing non-lethal, or favorable risk prostate cancer with active surveillance, potentially lethal, or high-risk prostate cancer generally requires multimodality treatment. These multimodality treatments could be surgery with or without radiation and androgen deprivation therapy (ADT), or radiation plus ADT with or without brachytherapy. This complicates the assessment of QOL, because these outcomes depend on the combination and timing of modalities.
Level One Evidence from ProtecT
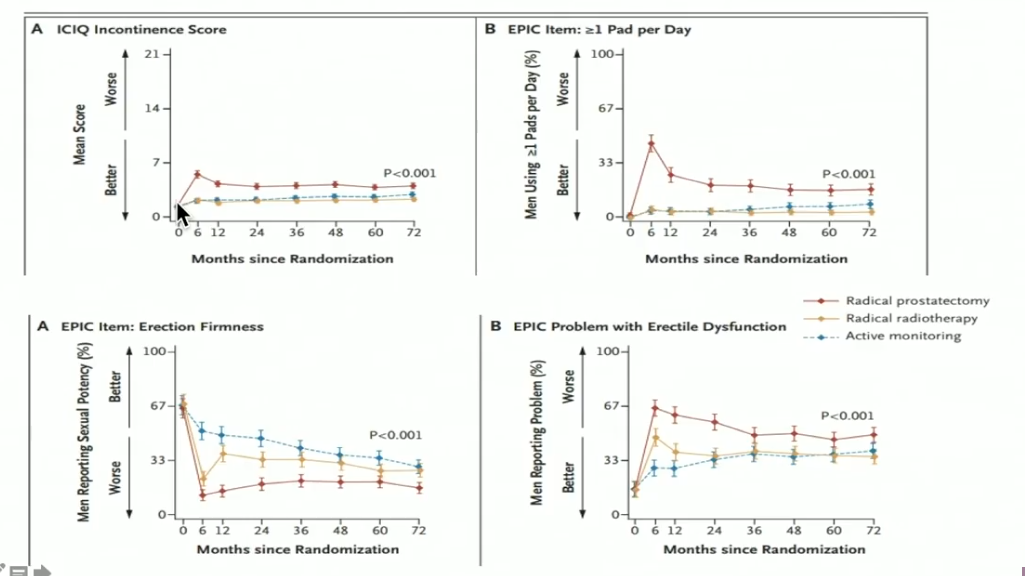
Dr. Crook reviews data from the Prostate Testing for Cancer and Treatment (ProtecT) trial, a prospective randomized trial that ran from 1999-2009, included 1643 patients with screen-detected prostate cancer. The trial compared radical prostatectomy (RP), radiation, and active monitoring. The figure to the left displays the prostate cancer specific mortality, distant metastases, and disease progression results.
The ERBT arm saw the worst bowel function symptoms at 6 months, but similarly to the urinary and sexual scores, those symptoms improved with the addition of ADT. However, hematochezia continued to be a problem for these patients. Furthermore, bowel bother and fecal incontinence recovered in all modality arms.
The Origin of Tri-modality Treatment
In 2009, Nelson N. Stone, MD and Richard G. Stock, MD, authored an article in the British Journal of Urology that looked at the effect of a high biologically equivalent dose (BED) on Gleason 7-10 disease treated with brachytherapy. This article became the basis for “tri-modality” treatment, or in other words, a brachytherapy, EBRT, and ADT combination.
This was a multi-center trial of over 1000 patients with a median follow-up period of 46 months. Approximately 80% of patients had Gleason score (GS) 7 prostate cancer, and 20-25% patients had a GS of 8-10. All received low-dose rate (LDR) brachytherapy.
Sixty-two percent of subjects received ADT for a median duration 4 months, while 58% received EBRT. For those who received EBRT in addition to brachytherapy, the median BED was 209 Gy, versus 155 Gy in those who received brachytherapy alone.
The 5-year freedom from biochemical failure (5-yr-FFBF) rate was higher in those receiving a BED > 200, which was displayed even more prominently in those with a GS of 8-10 (5-yr-FFBF with <200 Gy: 52%; 5 yr-FFBF with > 200 Gy: 86 – 90- %).
However, even with a BED of >220 GY, patients with ADT is still needed for those with GS 8-10.
The 5-yr-FFBF rate was 96%, with the addition of ADT, versus 78% for those without. ADT. There were also indications of improvement in rate of development of metastases.
Level One Evidence from ASCENDE-RT
Dr. Stone’s and Dr. Stock’s report was the impetus for the definitive Canadian randomized trial, Androgen Suppression Combined with Elective Nodal Dose Escalated Radiotherapy (ASCENDE-RT).
Dr. Crook reviewed this Randomized, Phase III study of 398 patients with a follow-up period of 5-11 years. Two thirds of the patients were in the high-risk category, while the remainder were classified as high-tier intermediate risk. All received one year of ADT and Pelvic Intensity Modulated Radiation Therapy (IMRT). Then, they were randomized to either the LDR brachytherapy arm, providing a boost of an additional 115 Gy, or the external beam radiation therapy (DE-EBRT) arm, which continued the external beam to a total of 78 Gy.
Nine years after start of ADT treatment, progression free survival (PFS) for the DE-EBRT arm was 62.4 %, versus 83.3% PFS for those treated with LDR-PB. The absolute difference between the two arms at 9 years was 20.95%
Looking at only the high-risk subjects at 9 years, the LDR-PB arm had a progression free survival rate of 78 percent, versus 58.2 % in the DE-EBRT arm. Randomization to the DE-EBRT arm (78 Gy) versus the LDR-PB (total of 115 Gy) was associated with a 2 times greater risk of biochemical failure at 6.5 years.
Quality of life data was not collected following a patients’ failure. So, toxicity of salvage was not a factor when comparing the two arms. However, after 5 years, prevalence of grade 3 genitourinary and gastrointestinal toxicity was higher in the LDR brachytherapy group, while the 5-year sexual dysfunction was similar to baseline, but actually better in the LDR-PB arm.
Comparing Modalities for High-Risk Prostate Cancer in a Prospective Database
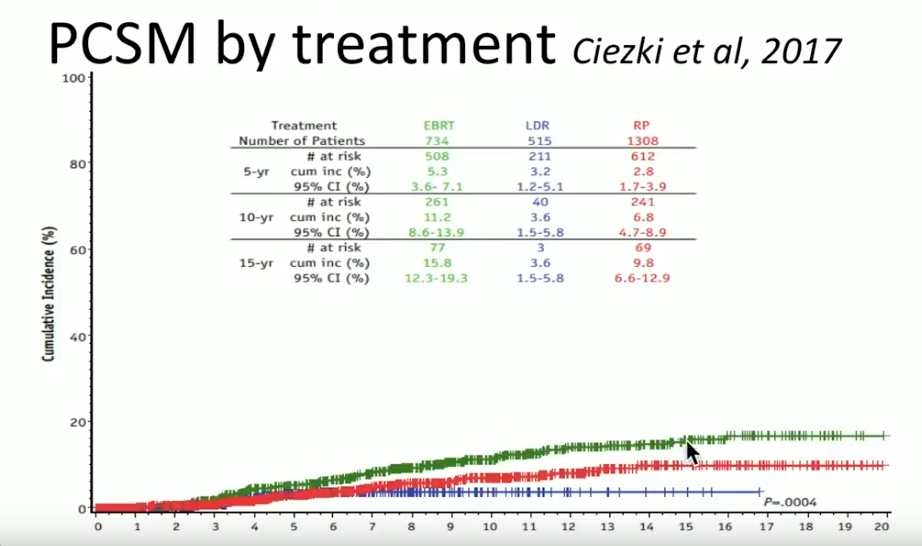
A prospective database from the Cleveland Clinic reported on 2557 patients treated from 1996-2012, with a median follow up of 63 months. Out of those patients, 734 received EBRT with or without ADT, 1308 had radical prostatectomy (RP) with or without EBRT, and 515 had LDR brachytherapy with or without ADT.
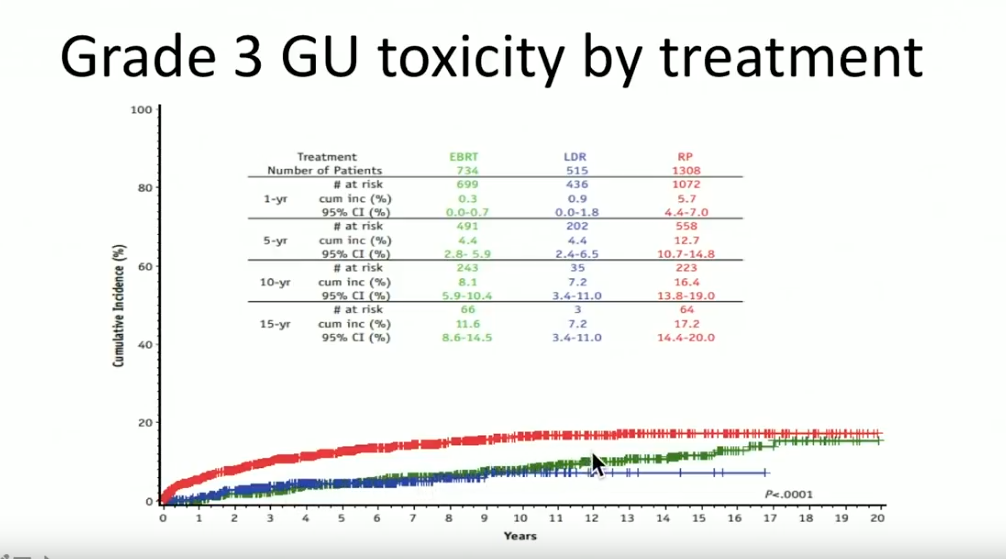
Overall, Dr. Crook claims that the tri-modality of ADT, EBRT, and brachytherapy is the most effective form of treatment for high-risk prostate cancer patients. Although clinicians must understand adding EBRT to brachytherapy may increase bowel symptoms and GU toxicity, improving techniques, like attention to sagittal imaging, can mitigate those effects.