Daniel P. Petrylak, MD, presented “Immunotherapy for Castrate-Resistant Prostate Cancer” during the 24th Annual Southwest Prostate Cancer Symposium on April 13, 2019 in Scottsdale, Arizona.
How to cite: Petrylak, Daniel P. “Immunotherapy for Castrate-Resistant Prostate Cancer” April 13, 2019. Accessed Nov 2024. https://dev.grandroundsinurology.com/immunotherapy-for-castrate-resistant-prostate-cancer/
Immunotherapy for Castrate-Resistant Prostate Cancer – Summary:
Daniel P. Petrylak, MD, analyzes the current status of immunotherapy for prostate cancer, reviewing available options, emerging combination therapies, and immunotherapy clinical trials. He also emphasizes the impact of PD-L1 expression, microsatellite instability, and other mutations in prostate cancer on patient responses to immunotherapy.
Immunotherapeutic Options for Prostate Cancer
Prostate cancer is inherently immunogenetic, and therefore immunological interventions can induce protective antitumor responses. The two main types of immunotherapy for prostate cancer are: 1) sensitizing the immune system to different antigens; and 2) uninhibiting the immune system, or checkpoint inhibition.
FDA-Approved Options
Sipuleucel-T is an FDA approved immunotherapy treatment option for prostate cancer.. However, Sipuleucel-T has been controversial because of the disconnect in overall survival (OS) and progression free survival (PFS). These differences may indicate the best time to use Sipuleucel-T in the treatment of prostate cancer.
Beyond PD-1 and PD-L1 Checkpoint Inhibitors
Checkpoint inhibitors are another type of immunotherapy that may improve prostate cancer outcomes. Many checkpoint inhibitors target PD-1 and PD-L1. However, CTLA-4 is another checkpoint protein that can be targeted by checkpoint inhibitors. Ipiliumumab is an example of a CTLA-4 inhibitor. The FDA approved ipilimumab for melanoma and other solid tumor cancers.
A phase 3 study of ipilimumab, CA184-043, investigated post-docetaxel metastatic-castration resistant prostate cancer (mCRPC) patients. These patients were given one dose of radiation and then ipilimumab or placebo. Results of this study did not show a significant increase in overall survival (OS), but an interesting pattern formed. Patients who benefited from ipilimumab had bone-only disease. Patients with visceral metastases did worse in this study. Nonetheless, these results show the importance of patient selection in trials and drug choice.
PD-L1 Expression in Prostate Cancer
In hormone sensitive, radical prostatectomy specimens, about half are found to have PD-L1 expression on prostate cancer cells. Findings like this suggest that hormone status may be correlated with PD-L1 expression. Similarly, patients who progress on enzalutamide have significantly increased PD-L1 dendritic cells in the blood compared to patients progressing on treatment. This may suggest a benefit of hormone therapy followed by anti-androgens. However, DNA mutations and microsatellite instability may provide clarity in understanding the best role for checkpoint inhibitors in prostate cancer treatment.
A 2019 trial by Abida et al. investigated 1033 cancer patients and found that 3.1% of patients had microsatellite instability. One potential option for treating prostate cancer patients with microsatellite instability is pembrolizumab. Pembrolizumab is approved for any unresectable or metastatic solid tumors with microsatellite instability that have progressed following prior treatment. CDK12 is another class of mutations that leads to genomic instability, gene fusions, and neoantigens. With an increased number of antigens on the prostate cancer cells, the cancer becomes more recognizable to the immune system and potentially responsive to checkpoint inhibition therapy.
Combination of Checkpoint Inhibitors
Identifying optimal combinations of checkpoint inhibitors may also result in better patient outcomes. For example, the combination of nivolumab and ipilimumab is approved in renal cell carcinoma. At ASCO-GU 2019, a study investigating this combination in prostate cancer presented results. This open-label, multicenter, phase 2 study (NCT02985957), CheckMate 650 trial shows responses to therapy, but a more robust response would be more encouraging. However, only about a quarter of patients in the study received the full dose of treatment due to toxicity, which may have impacted results. Despite this, patients with DNA damage repair mutations and high tumor mutation rates had better responses.
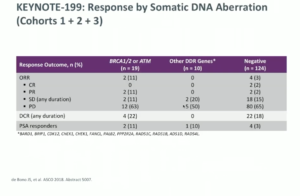
Similarly, the KEYNOTE-199 study of pembrolizumab in mCRPC took five groups of patients and separated them into those receiving enzalutamide vs. those who did not, then those with high PD-L1 status vs. PD-L1 negative, and finally those with bone metastasis, no measurable disease, and any PD-L1 status.
Regarding the antitumor activity in the cohorts relating to enzalutamide, the objective response rates were not particularly high. PD-L1 positive patients did slightly better overall, but the reasons are not well understood. Furthermore, DNA damage repair mutations were found to influence overall response rate and PSA response rate.
Additionally, the combination of checkpoint inhibitors and poly ADP ribose polymerase (PARP) inhibitors may provide better responses to immunotherapy. The KEYNOTE-3665 study evaluated the combination of pembrolizumab and olaparib in prostate mCRPC patients. This combination is moving to phase 3 trials because the combination was generally well tolerated and had promising activity in molecularly unselected mCRPC patients previously treated with chemotherapy and second-generation hormonal therapies.
Conclusion
In summary, it is suggested that physicians use immunotherapy early in the disease course of CRPC, check all patients for microsatellite instability, and utilize DNA repair mutations to predict responses to immunotherapy. It is important to keep in mind that PSA levels may not decline when evaluating patients on these drugs. The overall outcome for patients treated with immunotherapy may be survival.
About the Southwest Prostate Cancer Symposium
The Southwest Prostate Cancer Symposium (SPCS) is a multi-day conference that seeks to educate urologists, radiation oncologists, medical oncologists, and other healthcare professionals involved in the treatment of prostate cancer. The topics focus on current technical aspects of diagnosis and treatment of localized and advanced disease, particularly regarding imaging, technology, and training in the related devices. Dr. Petrylak presented this lecture during the 24th SPCS in 2019. In 2020, the 25th SPCS will also offer training sessions involving imaging, scanning, and prostate cancer treatment-related devices on site. Please visit this page in order to register for future SPCS meetings.
ABOUT THE AUTHOR
Daniel P. Petrylak, MD, is currently Director of Genitourinary Oncology, Professor of Medicine and Urology, Co-Leader of Cancer Signaling Networks, and Co-Director of the Signal Transduction Program at Yale University Cancer Center in New Haven, Connecticut. He is a recognized international leader in the urology field. He earned his MD at Case Western Reserve University School of Medicine in Cleveland Ohio. He then went on to complete his Internal Medicine Residency at Albert Einstein College of Medicine/Jacobi Medical Center in the Bronx, and his fellowship at Memorial Sloan Kettering Cancer Center in New York.
Dr. Petrylak has served as principal investigator (PI) or co-PI on several SWOG clinical trials for genitourinary cancers. Most notably, he served as the PI for a randomized trial that led to the FDA approval of docetaxel in hormone refractory prostate cancer. He also helped to design and served as PI for the SPARC trial, an international registration trial evaluating satraplatin as a second-line therapy for hormone refractory prostate cancer.
Dr. Petrylak served on the program committees for the annual meetings of the American Urological Association from 2003-2011, and for the American Society of Clinical Oncology from 1995-1997 and 2001-2003. He also has served as a committee member for the Devices and Immunologicals section of the FDA. He has published extensively in the New England Journal of Medicine, Journal of Clinical Oncology, Journal of the National Cancer Institute, Cancer Research, and Clinical Cancer Research.