Scott Eggener, MD, and E. David Crawford, MD discuss an abstract presented during the American Urological Association’s 2019 Annual Meeting on May 5, 2019, in Chicago, Illinois, in this presentation titled “Pivotal Trial of MRI-Guided Transurethral Ultrasound Ablation in Men with Localized Prostate Cancer.”
How to cite: Eggener, Scott and Crawford, E. David. “Pivotal Trial of MRI-Guided Transurethral Ultrasound Ablation in Men with Localized Prostate Cancer” May 8, 2019. Accessed Apr 2024. https://dev.grandroundsinurology.com/pivotal-trial-of-mri-guided-transurethral-ultrasound-ablation-in-men-with-localized-prostate-cancer/
Pivotal Trial of MRI-Guided Transurethral Ultrasound Ablation in Men with Localized Prostate Cancer
– Summary:
Scott Eggener, MD, reviews the findings from a phase II international, multi-institutional trial evaluating a new minimally-invasive technology for prostate tissue ablation. He covers the primary safety and efficacy endpoints of the trial, as well as future directions for this technology.
Dr. Eggener’s Late-breaking Abstract at the AUA’s 2019 Annual Meeting
Several important findings and presentations came out of the American Urological Association’s (AUA’s) 2019 Annual Meeting. One notable presentation is this late-breaking abstract involving a new technology called the MRI-guided Transurethral Ultrasound Ablation (TULSA) system. Scott Eggener, MD, Professor of Surgery and Radiology at the University of Chicago Medicine, presented this abstract. He is the principal investigator of the phase II international, multi-institutional trial evaluating this technology. During this video, he shares his findings with the Grand Rounds in Urology audience.
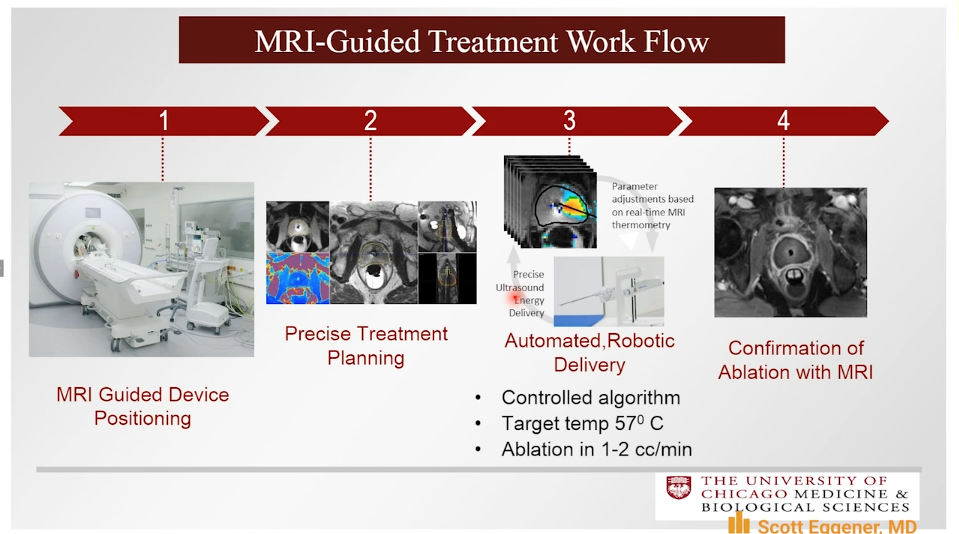
MRI-Guided Transurethral Ultrasound Ablation
The TULSA system employs a transurethral device that continuously rotates and emits directional ultrasound and uses active MRI thermometry feedback control in order to ablate the prostate.
The TULSA-PRO Ablation Clinical Trials (TACT) pivotal study included 115 patients from 13 institutions and 5 countries. Of these patients, 67% had intermediate-risk and 33% had low-risk prostate cancer. Patients received TULSA with the intent of whole-gland ablation. The trial met its primary efficacy endpoints with 96% of patients achieving a PSA decrease of 75% or more, 65% of patients with no evidence of disease on biopsy, and 79% of men with baseline Grade Group 2 disease had no evidence of Grade Group 2 disease on biopsy at 1-year follow-up.
The safety profile from this trial appears favorable towards this technology. Upon 1-year follow-up, 96% of patients maintained or returned to baseline continence level, and 75% of patients maintained or returned to erections sufficient for penetration.
In the future, there will be a protocol-mandated 5-year follow up with these patients. Also, there are ongoing discussions with regulatory authorities in the United States and abroad regarding potential approval of this technology for prostate ablation.
Following Dr. Eggener’s review of this data, he and E. David Crawford, MD, confer about the plans for long-term follow-up from this trial, clarifying the differences between TULSA and high-intensity focused ultrasound (HIFU), and the possibility of using TULSA for targeted focal therapy.