Point Counterpoint in ADT—Agonists versus Antagonists
How to cite: Karsh, Lawrence & Keane, Thomas. “Point Counterpoint in ADT—Agonists versus Antagonists” January 27, 2018. Accessed. https://dev.grandroundsinurology.com/Point-Counterpoint-in-ADT-Agonists-versus-Antagonists/
Summary:
Lawrence Karsh, MD, FACS, argues that agonists are effective, sustainable androgen deprivation therapy (ADT) options, and the belief that agonists present a high cardiovascular (CV) risk could be due to selection bias in trials. Conversely, Thomas Keane, MD, argues that patients treated with antagonists have lower CV risk and are more responsive to ADT than those treated with agonists.
To further expand your knowledge of prostate cancer treatment with ADT, visit the Androgen Deprivation Therapy Next Generation Learning Center.
Point Counterpoint in ADT—Agonists versus Antagonists – Transcript
Click on slide to expand
Argument for Agonists
GnRH Agonist vs. Antagonist Which One?
We’re going to look at agonist versus antagonist, which one? I think they’re both good drugs. I use them both, but I’m going to try to make the case for the agonist.
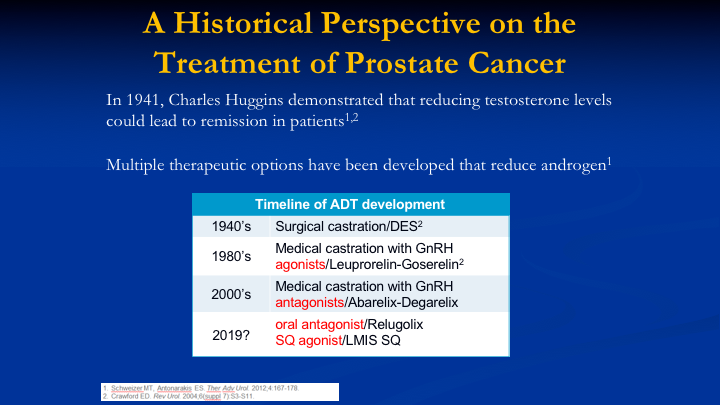
A Historical Perspective on the Treatment of Prostate Cancer
The historical perspective of treatment with ADT is well known to this audience. Surgical castration is still considered the gold standard for suppression of testosterone, and then we had the agonists come on as well as the antagonists, and probably in 2019 or 20, we’re going to have our first oral antagonist if it meets all of the endpoints in the trial, relugolix and another subcutaneous agonist from Foresee Pharmaceuticals.
Clinical Impact of Low Testosterone Levels: Two Peer-Reviewed Articles
So these are the papers of Morote and Perachino, which talked about the clinical impact of low testosterone levels.
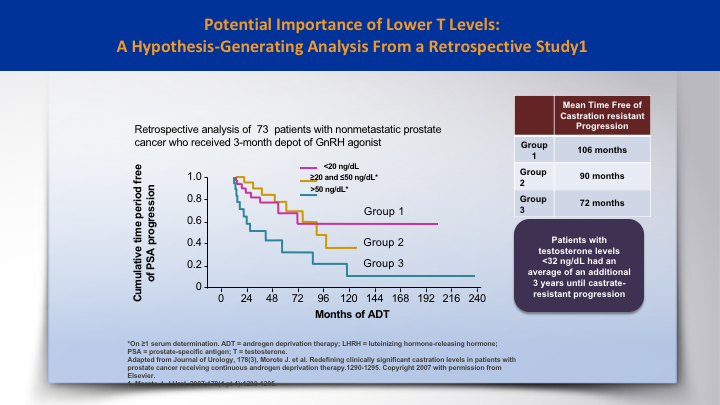
Potential Importance of Lower T Levels: A Hypothesis-Generating Analysis from a Retrospective Study 1
And the Morote paper was a hypothesis generating paper that looked at 73 patients. And if the testosterone was lower than 20, then those patients had an improvement, a three-year improvement in time to castration resistance, as opposed to those who were above 50.
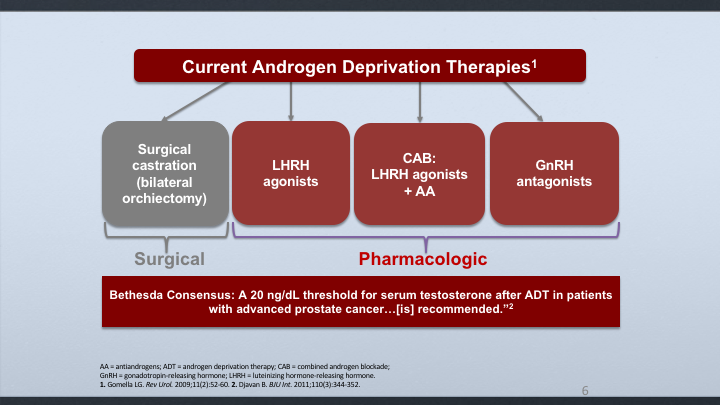
Current Androgen Deprivation Therapies
And this led to a consensus by the Bethesda group, which is a notable group of docs, experts in the field, that came up with a threshold of 20 nanograms/deciliter for considering castration.
Current Androgen Deprivation Therapies
And this led to a consensus by the Bethesda group, which is a notable group of docs, experts in the field, that came up with a threshold of 20 nanograms/deciliter for considering castration.
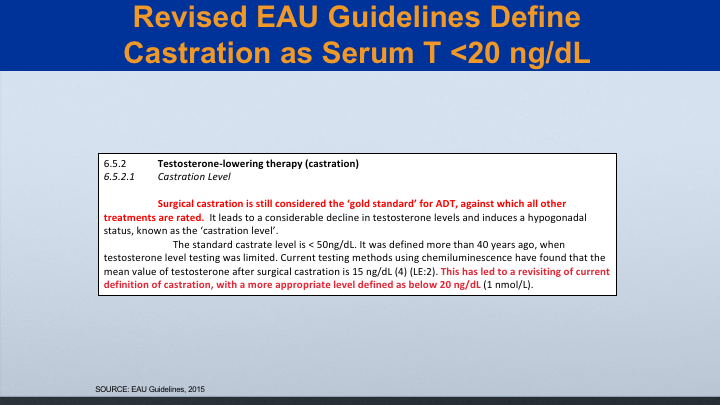
Revised EAU Guidelines Define Castration as Serum T <20 ng/dL
This was updated by the EAU in 2015, where they said that surgical castration is still considered the gold standard against which all other treatments are rated, and they also said that castration really should be below 20.
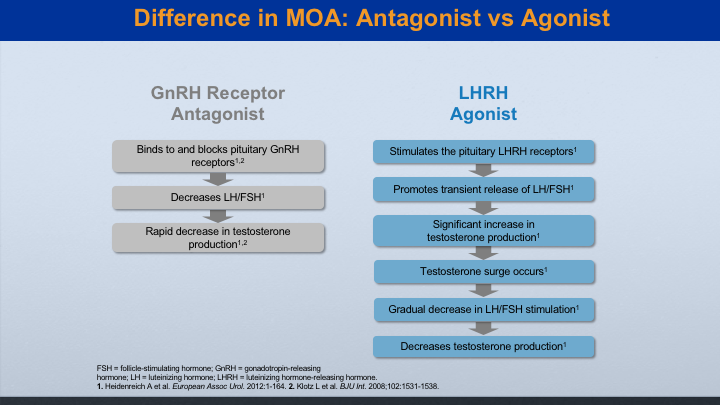
Difference in MOA: Antagonist vs Agonist
These are the different mechanisms, the antagonist versus the agonist. The antagonist works quicker. It shuts down the production of LH and FSH, and so that you’ll get probably decreased testosterone levels to castrate within about 2 or 3 days. The agonist actually stimulates before it suppresses the androgen, and in that time period you get a testosterone flare, which sometimes needs treatment.
GnRH Analogues 2018
We have different flavors of agonists. We’ve got daily, monthly, three, four, six, and twelve month, whereas the antagonist comes only in a monthly subcu injection.
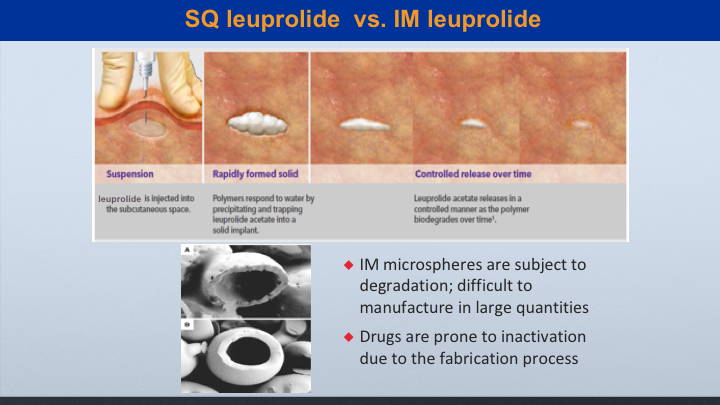
SQ leuprolide vs. IM leuprolide
This is when looking at subcu leuprolide, which is marketed as Eligard versus IM leuprolide, which is marketed as Lupron, the subcu leuprolide has a delivery package that creates a controlled release over time. So it can be more sustained. But even though the IM leuprolide is made of microspheres that can cause degradation, I’m going to show you some data to show that it’s still very effective.
GnRH agonists: Advantages
So the advantages of the agonists are that it can lower testosterone, the efficacy is comparable to orchiectomy, variable dosing, and when confronted with a decision or choice, patients really prefer injections to surgical castration.
GnRH agonists: Disadvantages
So some of the disadvantages are that the agonist can cause a surge and flare symptoms. As far as the microsurges and breakthroughs, I’m not sure how important that is. We now have a PR7 study showing that there really wasn’t a difference in intermittent or continuous therapy in patients that had non-metastatic disease, and of course the agonists have the hormonal side effects. Now, this was the thinking because of Morote and Perachino that testosterone couldn’t be reduced to comparable to orchiectomy, but I’m going to show you some data that shows that is really not true. These things are comparable.
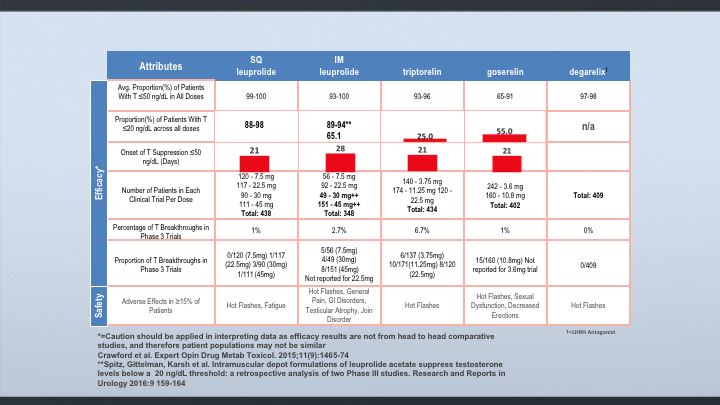
Attributes
When we look at the levels below 50, if you look at subcu leuprolide, IM leuprolide, and Degarelix, they all suppress it with a high percentage of patients. When it comes to suppressing below 20, subcutaneous leuprolide also was shown to have 88 to 98% of the patients were suppressed. In the IM leuprolide, if you looked at all of the data, it was really 65, but we did a retrospective analysis and a pooled analysis of the 4 and 6-month dosages, and I’ll show you that it is 89 to 94%. But the data for Degarelix is not available, and then when you look at the breakthroughs, subcutaneous leuprolide was about 1%. IM leuprolide 2.7%, and we’re at zero for breakthroughs on Degarelix.
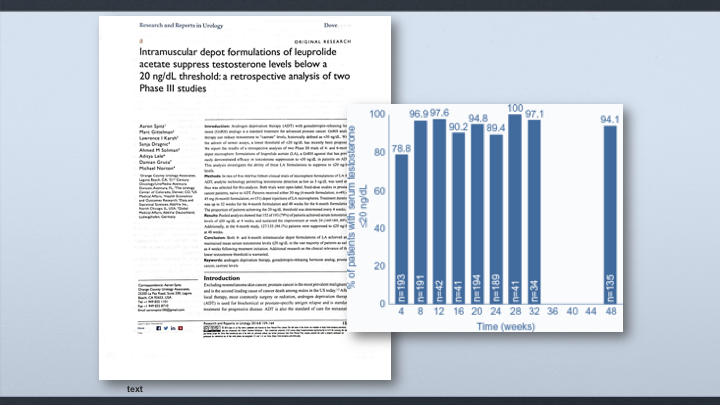
Intramuscular depot formulations of leuprolide acetate suppress testosterone levels below a 20 ng/dL threshold, a retrospective analysis of two Phase III studies
This is the retrospective analysis that we reported about a year ago showing that the four-month and six-month formulation of leuprolide, IM leuprolide, that we get about a 79% reduction down to 20 in the first four weeks. It goes up to 89% at 24 weeks, and then 94% at 48 weeks.
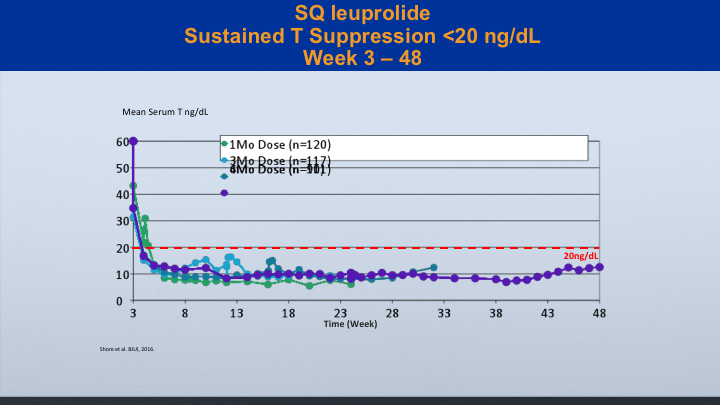
SQ leuprolide
And it’s true with the sq leuprolide. You can see the study that was reported by Neal Shore and associates showing good suppression below 20 with IM leuprolide
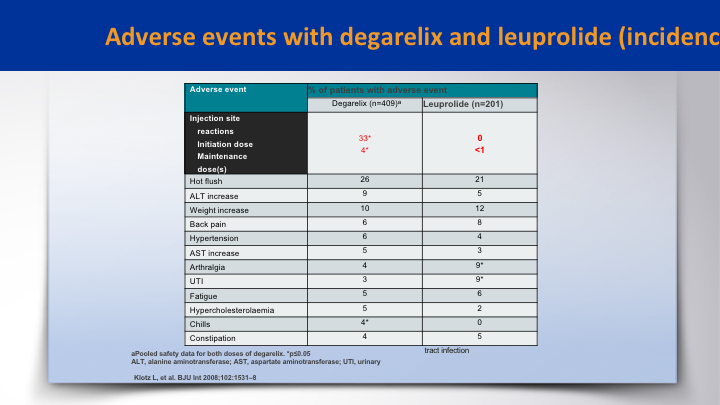
Adverse events with degarelix and leuprolide (incidence)
When it came to adverse events, it was pretty balanced between both groups. The difference was the initial skin site reactions which are higher in degarelix on the initial dose versus zero in the leuprolide group. This was in the CS21 study, the registration trial for degarelix. There’s probably a little higher ALT abnormalities in the degarelix and probably more arthralgias in the leuprolide.
FSH Story
What about the FSH story? What is that all about? So we had a paper by David Crawford and a number of other authors including Tom Keane. We know that FSH can serve as a mitogen for tumor growth that’s been shown by Ben Joseph and Radu and also it’s found in the vasculature of the prostate cancer cells. But there’s also this thing about it’s relationship to bone, and that it may stimulate rank ligand, which can induce osteoclastogenesis with bone loss and fractures, and also they hypothesize that FSH may even have something to do with memory loss.
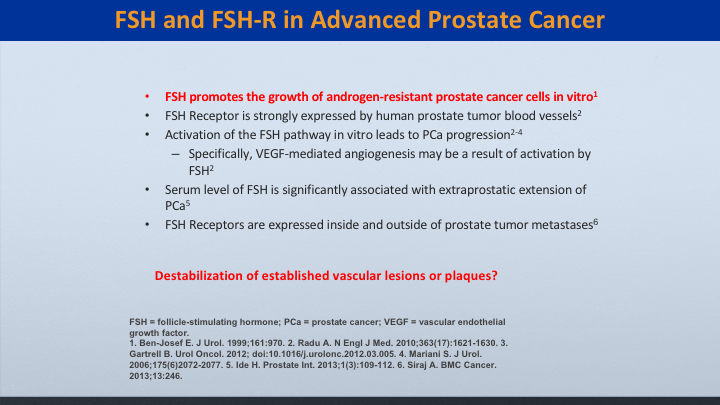
FSH and FSH-R in Advanced Prostate Cancer
We know that it promotes the growth of cancer, but does it really destabilize these vascular lesions or plaques?
The FSH Story
And so do we know all the facts with FSH? Do animal studies translate to humans, and is there more to it than just FSH in cardiovascular disease?
Poster MP47-05
Low Serum testosterone is Associated with Elevations in High-Sensitivity Cardiovascular Disease Biomarkers
This is an interesting paper looking at cardiovascular disease biomarkers, and it won the best poster award at the AU in 2016. What they did was look at the specific cardiovascular disease biomarkers, which are objective and specific for—that prognosticate things such as—so these markers will tell you if you have atherosclerosis, MI, cardiac failure, inflammation, dyslipidemia, and what they found was—they looked at it from a different approach because of the controversy of giving T for low-T patients, and the relationship between that and cardiovascular disease. But what they found was the lower testosterone, the higher these cardiovascular markers, and so it would be nice if we had in some of these prospective studies looking at when we’re comparing antagonists to agonists, whether or not there’s differences in these cardiovascular biomarkers.
CV Risk Factors and ADT
And we know that ADT does not cause the cardiovascular disease. It causes metabolic syndrome. This is a report from the European Society of Cardiology
NIH Public Access
And even in a paper support the use of Degarelix, Lorry Klotz and Matt Smith reported that in men with prostate cancer Degarelix and leuprolide had similar cardiovascular safety profiles. These observations suggest that the cardiovascular events associated with both agents result from hypogonadism rather than the direct drug effect.
Urologic Oncology
Here’s a paper that throws into question the studies that were used to support the use of degarelix, and it was published in Urologic Oncology in 2016, and what the author says is that the studies supporting the use of degarelix are criticized based on the selection bias in regards to the heterogeneous population described ad hoc analysis in the low statistical events and the presentation of selected bias that would appear to be favorable to the evaluated medication. In addition, those studies have not shown that there is any other point than to say that there’s an association with clinical benefit, and so they concluded that the flawed methodology of these publications make the existence to support the use of degarelix rather weak. This is a pretty critical article.
Article in Press
But this was one of the articles in support of degarelix and it was by Albertson. They looked at the cardiovascular morbidity associated between the agonists and the antagonists, and this is one of the things they found.
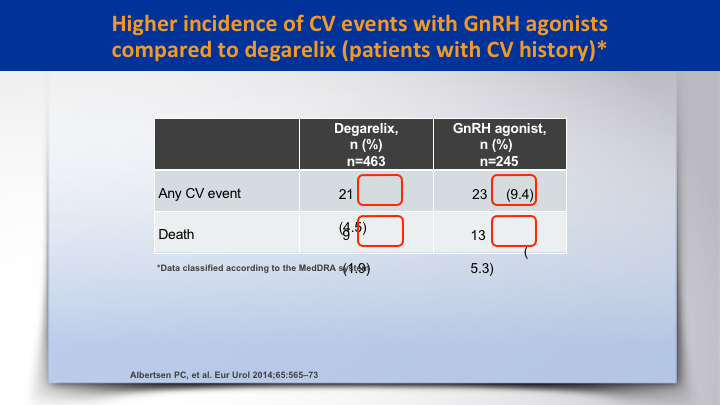
Higher incidence of CV events with GnRH agonists compared to degarelix (patients with CV history)*
There was a higher incidence of CV events with GnRH agonists compared to degarelix. It was double, 9.4% versus 4.5 for any CV event, and the death rate was higher in the agonist as opposed to degarelix 5.3 to 1.9.
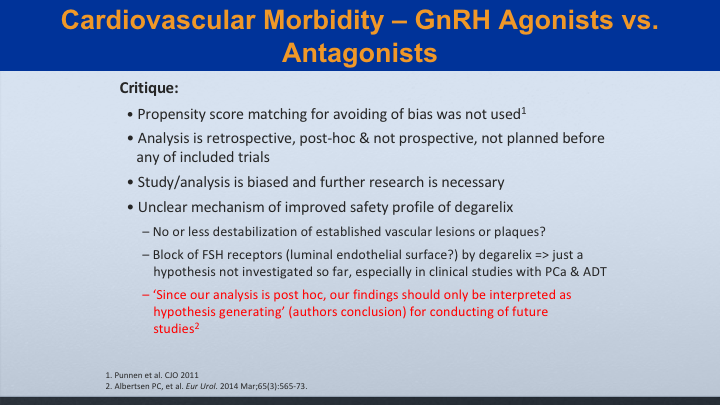
Cardiovascular Morbidity—GnRH Agonists vs. Antagonists
My critique is that there was no propensity score matching for avoiding the bias, and that wasn’t used. The analysis was retrospective. It was post hoc, and it was not pre-specified. The study analysis is biased, and further research is necessary, and there are unclear mechanisms on improved safety profile of degarelix. Does it really cause destabilization of these vascular lesions? And since our—this is from the authors themselves. Since our analysis is post hoc, our findings should only be interpreted as hypothesis generating for conducting future studies.
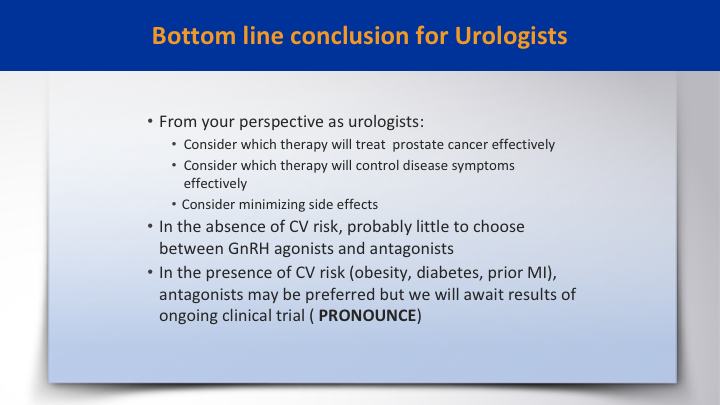
Bottom line conclusion for Urologist
The bottom line conclusion for urologists is from your perspective consider which therapy will treat prostate cancer effectively, and consider which therapy will control the disease effectively with minimal side effects, and in the absence of CV risk, there is probably little to choose from between the GnRH agonists and antagonists, and in the presence of CV risk, obesity, diabetes, prior MI, antagonists may be preferred, but we will await the results of the ongoing clinical trial.
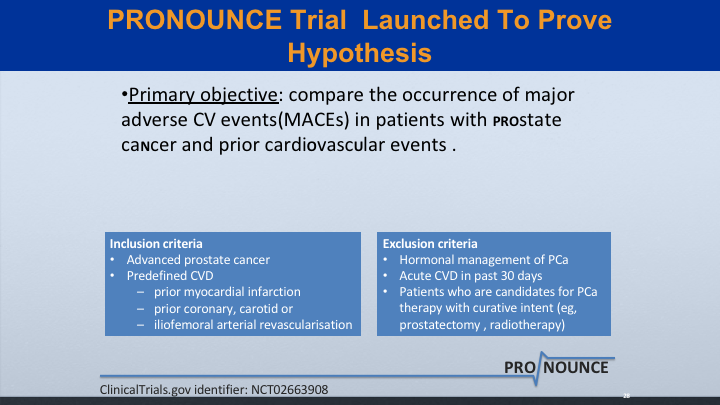
PRONOUNCE Trial Launched to Prove Hypothesis
And I give Ferring a lot of credit for launching this trial to prove the hypothesis. The primary objective is to compare the occurrence of major CV events (MACEs) in patients with prostate cancer and prior cardiovascular events.
Thank you!
So that’s what I have to say about the agonists. So thank you very much, and I’ll let Tom beat me up at this point.
Argument for Antagonists
Personalized ADT for the Specific Patient
So ADT must be personalized for the specific patient. There’s a number of things that you have to look at, cardiac status, obesity, testosterone levels before you start, monitoring the testosterone levels on a regular basis, which most urologists don’t do. FSH levels. What’s the degree of metastatic disease that the patient has? We’re not just trying to control testosterone. We’re trying to see if this is going to help treat somebody who has a rising PSA. That’s totally different from somebody who has a load of mets. We want to look at its use with chemotherapy and also there is some evidence of significant LUTS improvement.
Journal Article Review—Circulation Feb 2016
Again, in 2016 there was this article from Circulation, which suggests, which said cardiovascular events of androgen deprivation therapy for the treatment of prostate cancer is a real thing, and they gave us a mechanism by which we can look at our patients, assess the risk for cardiovascular disease, and actually do something about it.
Cardiovascular risk profile and ADT
So the real question here is is there a difference between agonists and antagonists, and let’s start with a cardiovascular risk profile and androgen deprivation therapy.
Preliminary trials show CARDIOVASCULAR event risk with GnRH antagonist treatment
Again, this is from the American Heart Association, and it is observational studies, and I’ll make the point now that it’s remarkable how in randomized, controlled trials we don’t tend to see as much difference. Yet, in observational studies the differences keep coming. Why is that? Well, study patients are just that. They’re study patients. They’re screened. They’re selected, and you’re quite right, Larry, they’re not going to put a load of significant cardiovascular disease patients in. They’re going to try and exclude some of those patients. That’s what happens when you do studies. When you do observational, you’re left with, well, I wonder if this is really true or not? So we do need that randomized trial. Ferring, get that trial done because we have to do it, but nonetheless, their conclusion was the preliminary trials show cardiovascular disease event risk with a GnRH antagonist treatment is 50% lower than with GnRH agonist treatment, and it’s interesting that there are very few studies, which have actually looked at cardiovascular side effects among the agonists themselves. There’s not a whole lot out there whereas there is a lot or, well, Ferring is to be congratulated for doing one of the major studies that actually does show a difference.
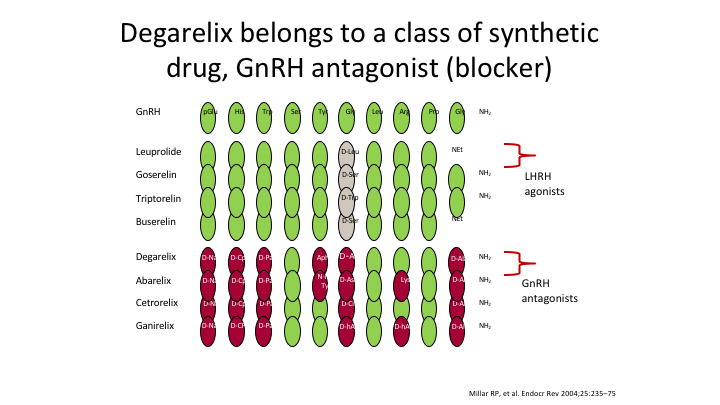
Degarelix belongs to a class of synthetic drug, GnRH antagonist (blocker)
Here’s the structure of the two compounds, totally different. It takes seven amino acids to make an antagonist. It takes one change to make an agonist.
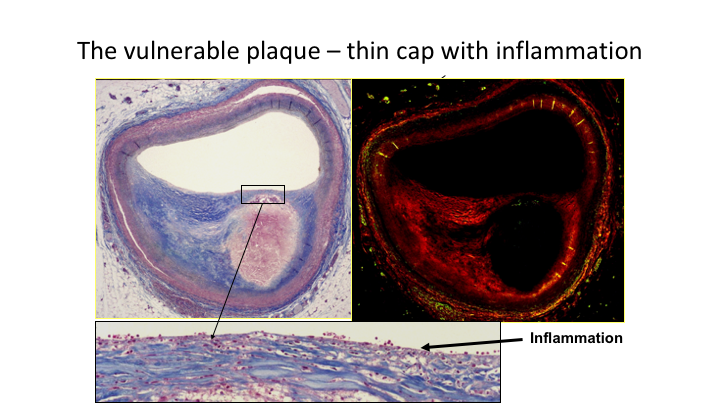
The vulnerable plaque—thin cap with inflammation
This is what we’re talking about. This is the heart of the situation if you like. This is your plaque, and here it is, you can see the more inflammation present the more unstable the plaque is.
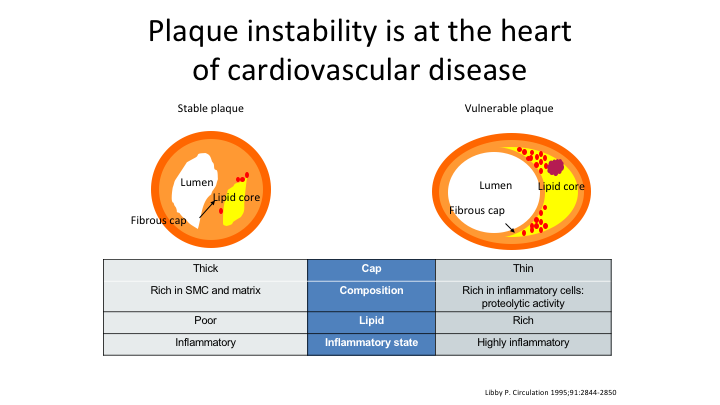
Plaque instability is at the heart of cardiovascular disease
As we look at plaque instability in terms of cardiovascular disease, you don’t want the thin plaque, you don’t want the inflamed plaque, you don’t want the plaque that has a lot of lipid in it.
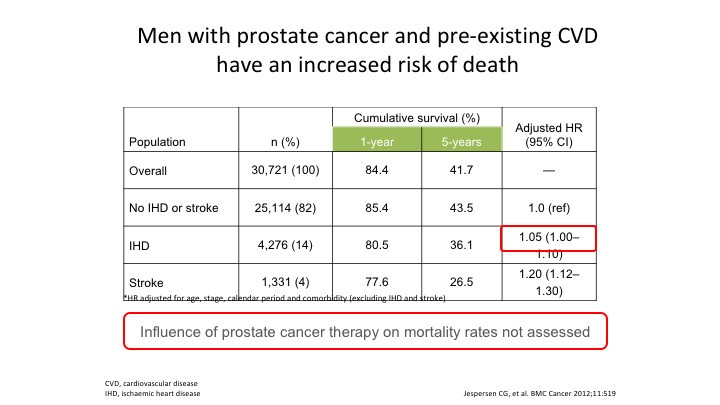
Men with prostate cancer and pre-existing CVD have an increased risk of death
Again, these are observational, I absolutely agree. This is Jasperson’s data who showed that men with prostate cancer and pre-existing cardiovascular have an increased risk of death when they go on any kind of castration treatment, and again if you–the influence of prostate cancer therapy on mortality rates in this study was not assessed.
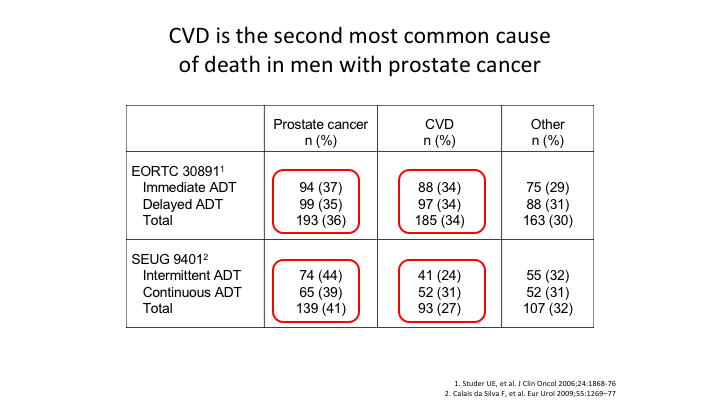
CVD is the second most common cause of death in men with prostate cancer
Then we look at the EORTC and 9401. Both of those once again show that cardiovascular disease is the second most common cause of death in men with prostate cancer.
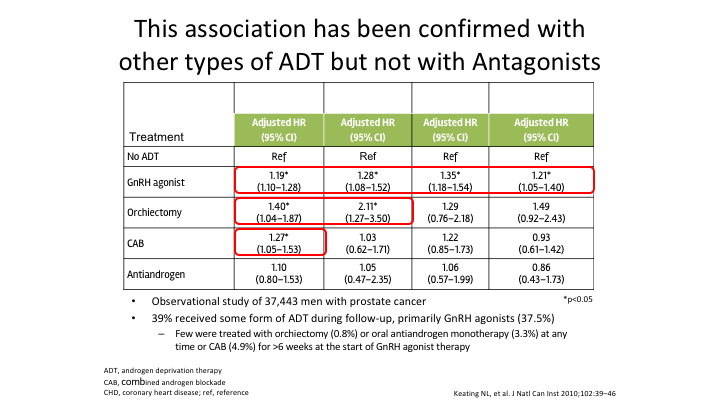
This association has been confirmed with other types of ADT but not with Antagonists
This association has been confirmed with other types of ADT. It has been seen with orchiectomy. It’s been seen with complete androgen ablation, and it’s been seen with the agonists. It has not yet maybe because there hasn’t been enough analysis done. It has not yet been confirmed with the antagonist.
The risk has been shown to be increased in older men and those with comorbidities
And then D’Amico even pointed out that men aged over 65 years of age receiving six months of ADT had shorter times to fatal MIs compared to radiotherapy alone, and again that was with the use of ADT, and at that time it was agonists that were used.
Based on the studies shown…
So if we look at this, based on what I’ve shown you, the actual increase in risk of cardiovascular disease in men treated with ADT be it orchiectomy, estrogen or GnRH agonist appears to be 20 to 25%, similar to the risk of men continuing to smoke. That’s a big number.
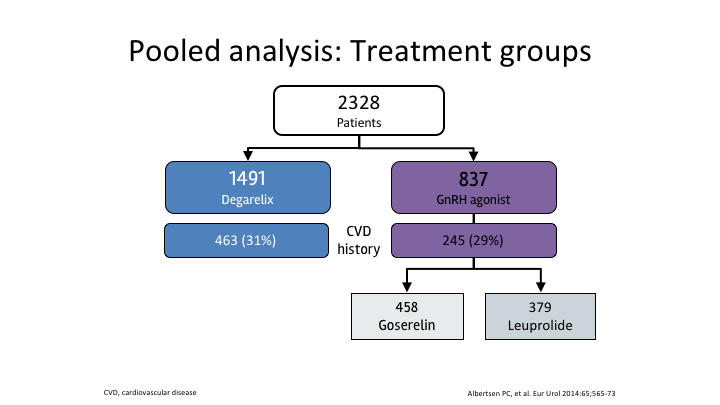
Pooled analysis: Treatment groups
So the pooled analysis, Alberton, yes, it was 2300 patients. There were nearly 1500 patients who received the antagonist. There were 837 who received the agonist, and they were fairly evenly split, and it did show lower cardiovascular events or death with degarelix for all patients, and once you went to the men with existing cardiovascular disease, that risk was significantly greater.
Overall survival
Then overall survival again was significantly greater in terms of the patients on the antagonist, and prostate cancer was not the cause of death in these patients because this was at one year, and one thing we have to state is that for cardiovascular risk, most of the risk is in the first year of treatment. It’s as they go on. It’s not as high, but as they start, it is. Why? Maybe it’s something to do with the induction.
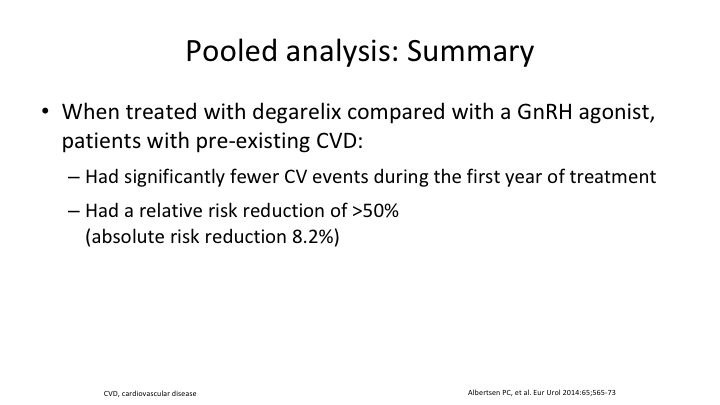
Pooled analysis: Summary
Maybe it’s something to do with the alterations in FSH. We need to do more to look at it. And in a pooled analysis summary, they had significantly fewer cardiovascular events during the first year of treatment if they were on the antagonist. And they had a relative risk reduction of over 50% and an absolute risk reduction of 8.2%.
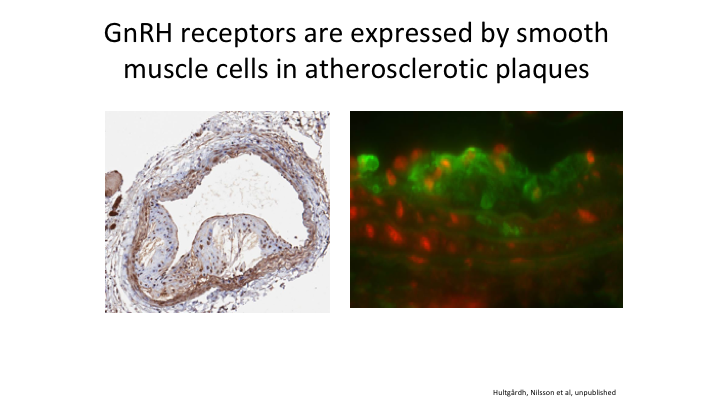
GnRH receptors are expressed by smooth muscle cells in atherosclerotic plaques
Here’s an example of an atheromatous plaque, and yes there are GnRH receptors located in that plaque. So how can you tell me it would be good to stimulate that in the initial month that you give an agonist when you don’t have to do that if you give an antagonist.
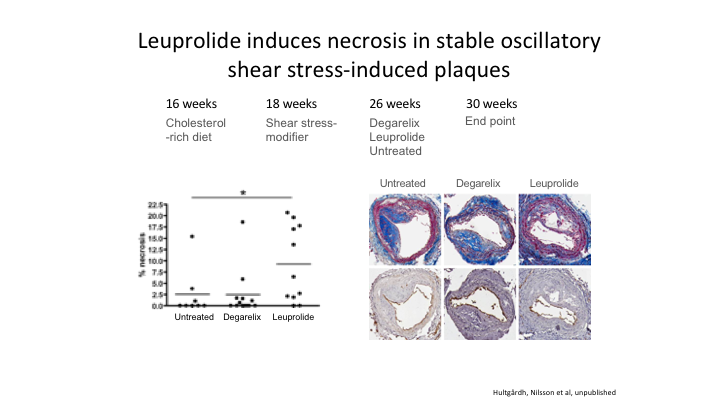
Leuprolide induces necrosis in stable oscillatory shear stress-induced plaques
Going back to basic science, this is from Professor Nilsson in Sweden. Basically, he had mice. Fed them a cholesterol-rich diet. At 18 weeks he induced an atheromatous plaque, and then he divided them into three groups, untreated, treated with leuprolide, or treated with the antagonist. And the ones who got the most inflammation, and you remember the slide I showed you inflammation is at the heart of the cardiovascular problem, the group on leuprolide had significantly more inflammation than either of the other two groups.
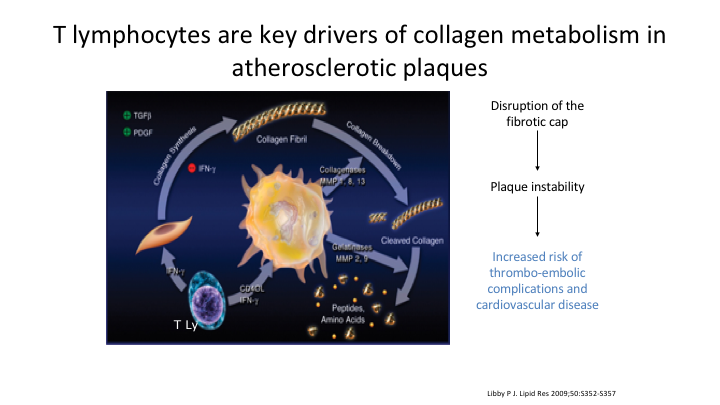
T lymphocytes are key drivers of collagen metabolism in atheromatous plaques
It’s the T lymphocytes which are the key drivers in collagen metabolism atheromatous plaques, we know that. We get disruption of the fibrotic cap. Basically it’s the T lymphocytes that they release. You cannot make normal collagen. It leads to instability and disruption.
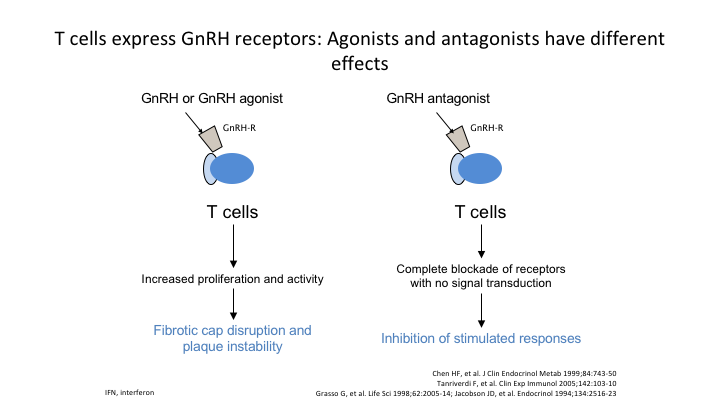
T cells express GnRH receptors: Agonists and antagonists have different effects
Here’s an example of the T cells, which express the GnRH receptors. If you use the agonist, it increases proliferation activity. If you use the antagonist, it basically shuts it down completely.
Potential mechanisms for differences in CV risk with different forms of ADT
And we know there are potential mechanisms for differences in the cardiovascular risk patterns with different forms of ADT. It could be metabolic changes. It may be GnRH receptor activation, or it may be differences in FSH levels. We’re not sure which, but we know it is probably one of these.
ADT has been associated with metabolic changes
Now, looking at the metabolic syndrome, we know metabolic syndrome is a disorder of energy utilization and storage diagnosed by the co-occurrence of any of the three listed in the first part of this slide. Metabolic syndrome increases the risk of developing cardiovascular disease, and we also know that androgen deprivation therapy does lead to insulin resistance, accumulation of subcutaneous fat and decreased lean body mass, increased glucose levels, and abnormalities in the lipid levels.
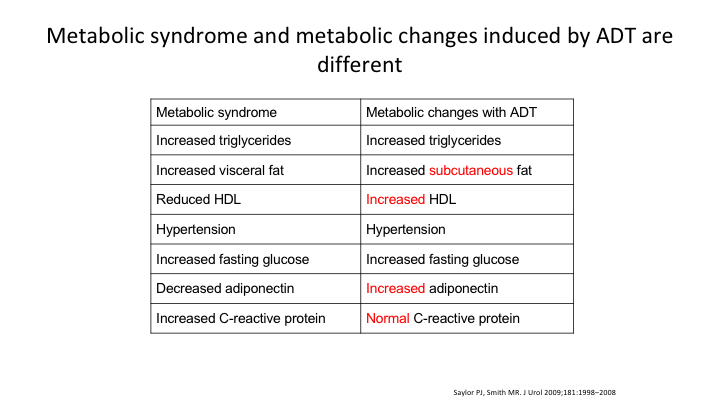
Metabolic syndrome and metabolic changes induced by ADT are different
When you look at what’s induced by ADT, there are subtle differences. There’s an increase in subcutaneous fat as opposed to visceral fat. There’s an increased HDL. Adiponectin is different. And normal—the C reactive protein is normal versus abnormal in patients with the metabolic syndrome. These are things that again are undergoing further investigation to see what’s going on.
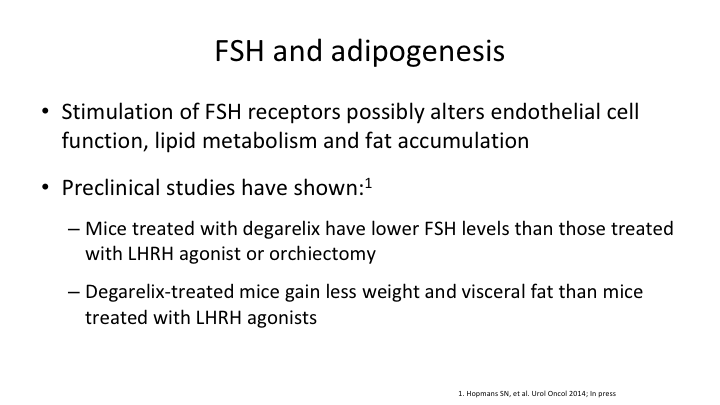
FSH and adipogenesis
Let me go back one. So preclinical studies have shown that mice treated with degarelix had lower FSH levels than those treated with LHRH agonists or orchiectomy. And the degarelix treated mice gained less weight and visceral fat than mice treated with LHRH agonist. So we have both pre- and real data to show you.
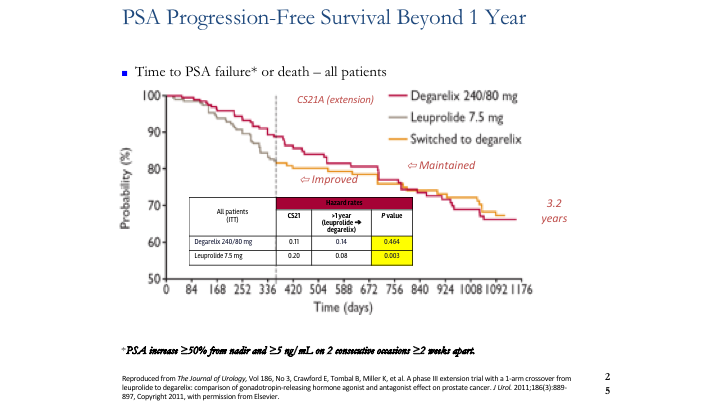
PSA Progression-Free Survival Beyond 1 Year
So let’s look at FSH, and there’s only one study that’s out there that actually looked at the results long term, and that is CS21. And like it or not, at the end of the first year there was a difference in the patients who had PSA progression as defined on that slide. Then the FDA said, okay, we need to switch everybody over to the agonist because we want to see bringing them out to three or four years is there going to be escape, and is there going to be problems? There weren’t, but what was noted was that the event rate changed from 0.22 back to 0.8. In other words, the number of patients failing ADT improved once they were switched from the agonist to the antagonist. This is the only study that has ever been done, randomized controlled trial, which showed that difference.
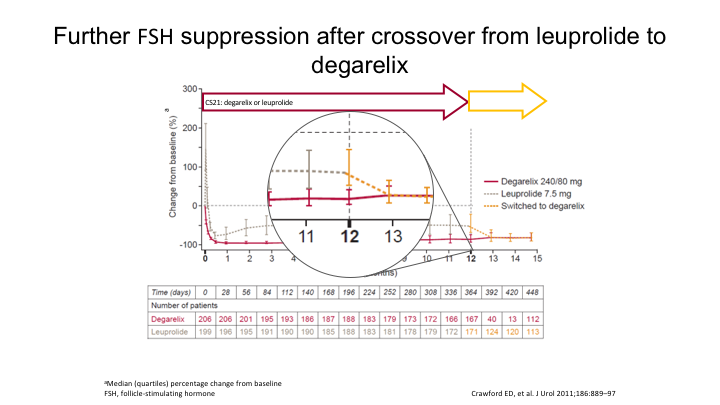
Further FSH suppression after crossover from leuprolide to degarelix
And interestingly, as they looked because they did keep T levels on everybody, and they also kept FSH levels, look what happened to the FSH. Here it is on the antagonist. Here it is on the agonist, and then when they were switched, there’s a further 63% reduction in the FSH. And again that is when we started to see things improving.
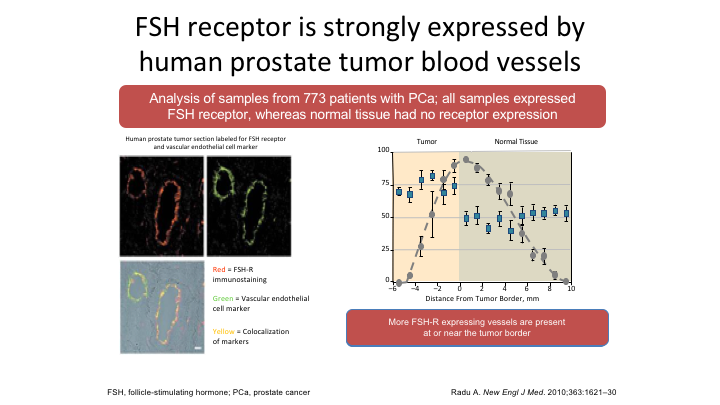
FSH receptor is strongly expressed by human prostate tumor blood vessels
So is FSH a real story? Well, again Larry quite clearly pointed out, and he’s correct, we don’t exactly know, but this is Radu’s paper in the New England Journal where he looked at samples taken from 773 patients with prostate cancer all samples express some FSH receptor whereas normal tissue they said didn’t have any, but in fact that was not true. There is a small amount of FSH receptor present in normal prostate tissue. But they did do co-localization assays, which showed that the FSH receptor and the VEGF receptor were in the same place, and then they looked again at specimens and found that as the metastatic areas grew so the FSH receptors increased, mets versus no mets, and then when they looked at the location of those FSH receptors they were at the leading edge of the metastatic deposit. That is what you need if you’re going to invade. You need a blood supply.
High Volume Metastatic Disease
Then let’s look at high-volume metastatic disease. The patients who did best in the CS21 extension study were patients who had a PSA of over 20, or the patients who had known metastatic disease. That is fact. The higher the volume of disease, the more benefit you got from being on the antagonist.
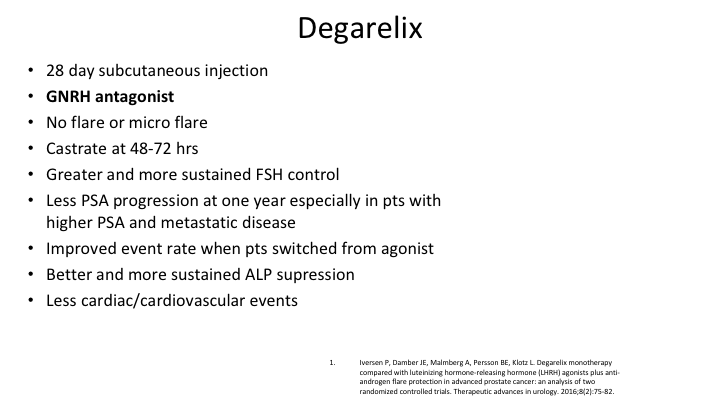
Degarelix
So if you summarize what’s going on here, degarelix 28-day subcutaneous injection, and it needs to be every 28 days, not every 31 days. It needs to be 28 days. It’s an antagonist. There’s no flare. There’s no micro flare. You’re castrate at 48 to 72 hours. There’s greater and more sustained FSH control as shown in a randomized controlled trial. There’s less PSA progression at one year in patients with higher PSA and metastatic disease as shown in a randomized trial. There’s better and more sustained serum alkaline phosphatase suppression, and there is less cardio and cardiovascular events.
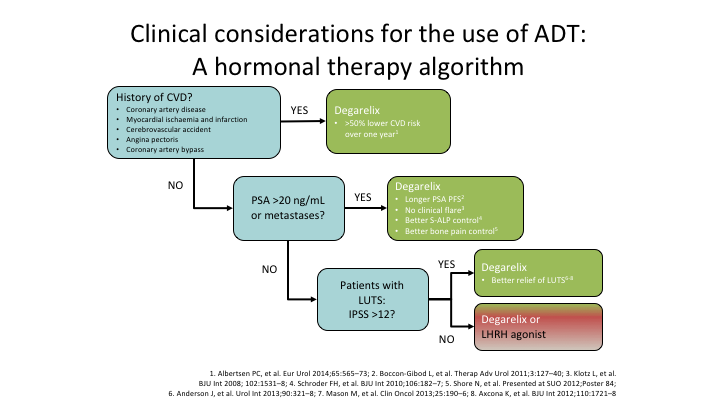
Clinical considerations for the use of ADT: A hormonal therapy algorithm
So if I’m going to give something to a patient, and not every patients needs to get degarelix, they don’t all need to get the antagonist, and you’re quite right, maybe it doesn’t matter if you’re controlling PSA rise, but I’m not sure we should be using it for PSA rise. But if you have a history of cardiovascular disease, why wouldn’t you put your patient on the antagonist, when all of the evidence, and admittedly it’s not a bucket load, but I have never seen any evidence that said the agonist was better. Same thing, if you’ve got a PSA of over 20 or you have metastatic disease, why wouldn’t you use the antagonist. Yes, there is a skin reaction. Fine. Maybe someday they will get it sorted out. And in the meantime I will say again, please get that study done. It’s been difficult to open worldwide. Ferring is aware of it. We need that study. So I use, and I haven’t used an agonist for a very long time because I do use the antagonist because the data that is out there so far is favorable to it. It’s not cut and dried, but I tend to look, and I want to go if there is an advantage I’m going to take it, and until somebody tells me there’s not, I’m going to stay taking it. Thanks very much.