Dr. Jehonathan H. Pinthus, MD, PhD, FRCSC, presented “Thromboembolic Complications in Bladder Cancer” at the 2nd Annual International Bladder Cancer Update on January 27, 2018 in Beaver Creek, Colorado
How to cite: Pinthus, Jehonathan. “Thromboembolic Complications in Bladder Cancer” January 27, 2018. Accessed Nov 2024. https://dev.grandroundsinurology.com/Thromboembolic-Complications-in-Bladder-Cancer/
Summary:
Jehonathan H. Pinthus, MD, PhD, FRCSC, explains factors that influence a bladder cancer patient’s risk of thromboembolic complications and prevention methods. His discussion includes the optimal timing of venous thromboembolism (VTE) prophylaxis after radical cystectomy, how cisplatin-based neoadjuvant chemotherapy effects thromboembolic events, and his opinion on current guidelines.
Reveal the Answer to Audience Response Question #1
Finish the sentence- Bladder cancer patients are at high risk for thromboembolic events, specifically:
- A. when treated with neoadjuvant cisplatin-based chemotherapy.
- B. when treated with primary cisplatin chemotherapy for metastatic disease.
- C. during the first week of post radical cystectomy.
- D. after discharge from post radical cystectomy.
- E. All of the above.
Reveal the Answer to Audience Response Question #2
Finish the sentence- Most thromboembolic events that occurred after radical cystectomy:
- A. occur within a week of the operation.
- B. occur after discharge home.
- C. are symptomatic.
- D. are associated with 15% mortality.
Reveal the Answer to Audience Response Question #3
Finish the sentence- VTE prophylaxis in bladder cancer patients is currently indicated:
- A. post radical cystectomy during the period of in-hospital stay and limited mobilization.
- B. for a period of 28 days post radical cystectomy.
- C. during cisplatin-based chemotherapy in ambulating patients.
- D. for all patients with advanced bladder cancer and pelvic lymphadenopathy.
Thromboembolic Complications in Bladder Cancer
Click on slide to expand
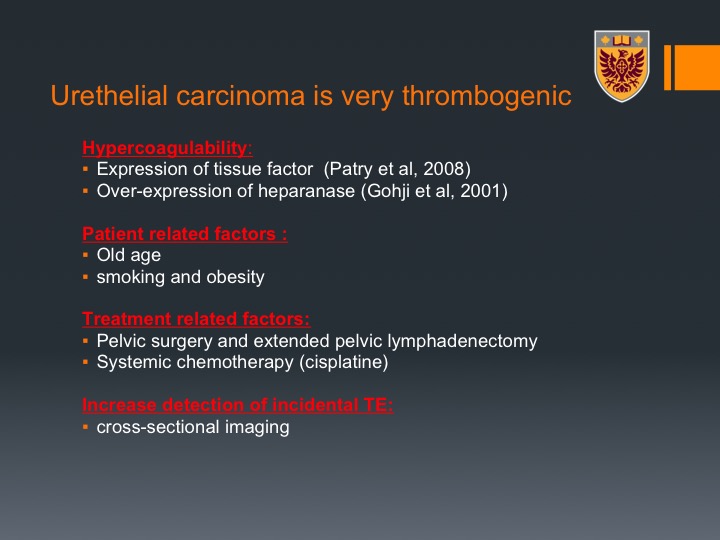
Urothelial carcinoma is a very thrombogenic tumor
Urothelial carcinoma is a very thrombogenic tumor and there are many reasons for that. First of all, it is a very hypercoagulable malignancy and this is just because of some biological properties, the tumor can express tissue factor and over expression of heparanase, which are actually pro-coagulable factors. Interestingly enough, and we’ll touch on that, these are factors that promote invasiveness and metastatic disease, and there is a correlation between patients who develop thromboembolic events and patients with advanced disease and worse prognostic factors.
There are patient-related factors. Patients are usually old, smoking, and obese all of those are risk factors for VTEs.
Treatment-related factors, obviously pelvic surgery with extended pelvic lymph node dissection is a significant risk factor and systemic chemotherapy in particular cisplatin, which is a thrombogenic agent and we are going to talk about that. And there is also increased detection of incidental PEs because we do a lot of cross-sectional imaging around the trajectories of the patient’s disease and treatment.
Tissue Factor
Tissue factor is expressed on cancer cells as well as on cancer cells derived microparticles, and cancer also induces inflammation that can further increase the tissue factor expression by host inflammatory cells that are within the tumor. The tissue factor localized coagulation factor seven to the cell surface of the activated clotting cascade, there is over expression of heparinase, which has been shown to be more common in patients with high-grade disease and locally advanced and metastatic bladder tumor, and this heparinase degrades the extracellular matrix and has pro-coagulant effects. So this is from the biological standpoint.
VTE complications in bladder cancer patients
And VTE complications in bladder cancer patients, if you will, can occur in three major trajectories of the disease. The most common one that we know is post radical cystectomy. But we have published recently on the neoadjuvant chemotherapy population and we will talk about that and as well patients with metastatic bladder cancer getting cisplatin-based chemotherapy and other chemotherapies.
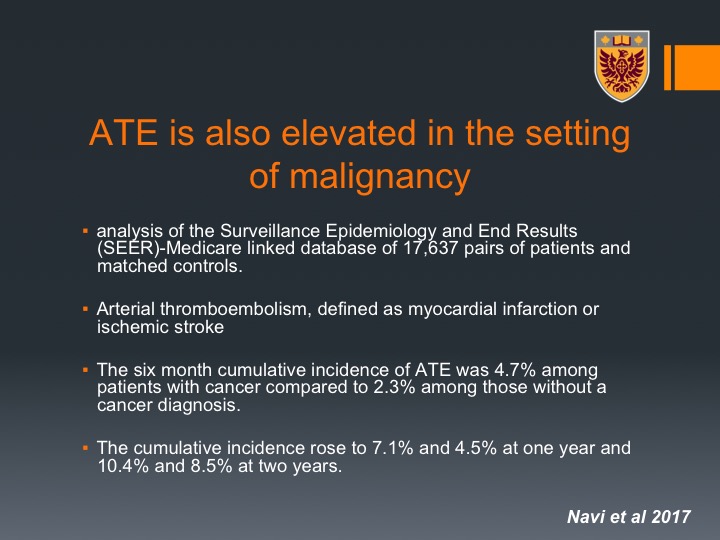
ATE is also elevated in the setting of malignancy
It is very important to know that it is not only venous thromboembolism, it is also arterial thromboembolism that can occur in this setting of malignancy and we tend to forget that. This is very important because they are very dangerous and they can be such as in myocardial infarction and ischemic stroke.
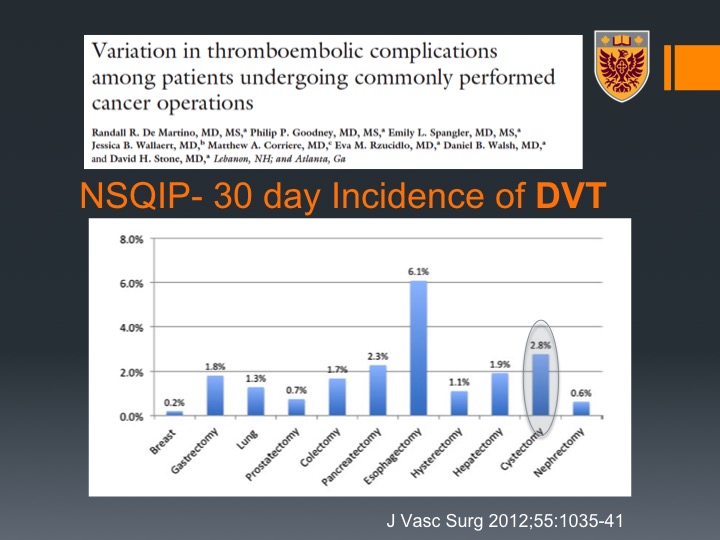
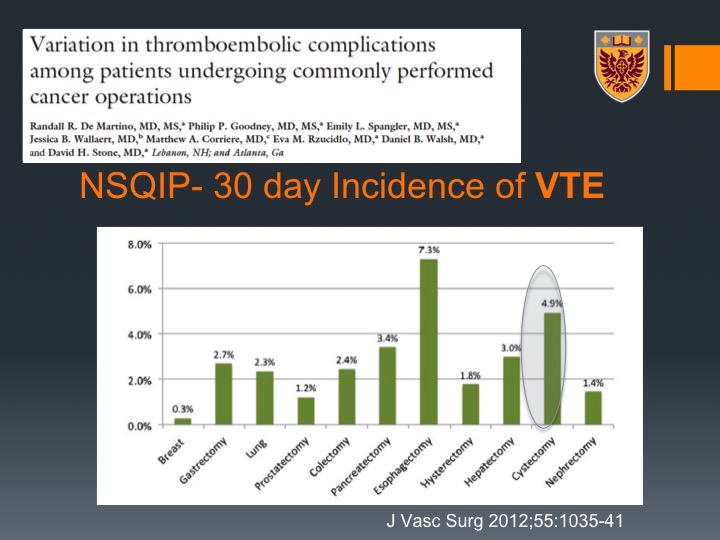
NSQIP-30 Day incidence of DVT
Now, if you look at the post radical cystectomy, this is data that was derived from the NSQIP database with about 44,000 patients with 11 types of malignancy and their associated cancers, and you can see that if you look at the 30 days incidence of DVT cystectomy is actually just secondary to esophagectomy, very high incidence of about 3%. If you look at PE it is even higher than esophagectomy, number one in terms of malignancies, in terms of PE development within 30 days of radical cystectomy. If you look at all VTE again 5%, and that is just secondary to esophagectomy. So bladder cancer and radical cystectomy for bladder cancer are very thrombogenic.
NSQIP-30 Day incidence of PE
If you look at PE it is even higher than esophagectomy, number one in terms of malignancies, in terms of PE development within 30 days of radical cystectomy.
NSQIP-30 Day incidence of VTE
If you look at all VTE again 5%, and that is just secondary to esophagectomy. So bladder cancer and radical cystectomy for bladder cancer are very thrombogenic.
EEU
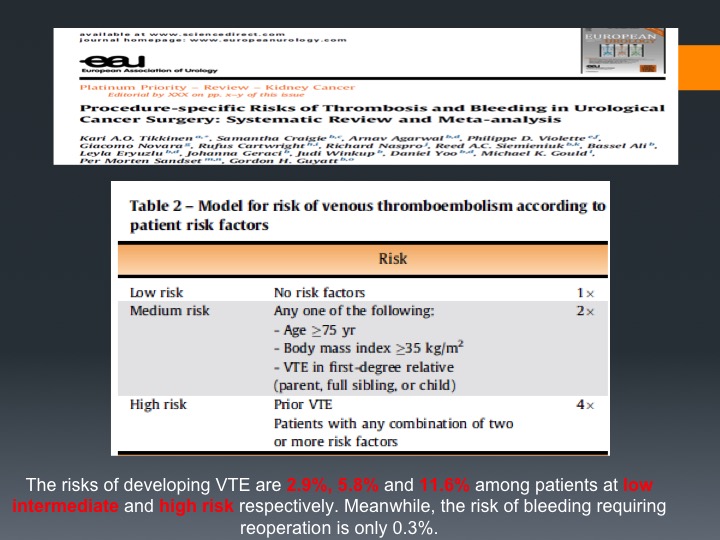
What’s interesting is that now there are new guidelines or to be guidelines at least in Europe and they estimate the risk of VTE to be up to 11.6% among high risk patients, and high risk they would define it as prior VTE or any combination of two or more of the risk factors respectively and the risk from bleeding by covering these patients (with VTE prophylaxis) is very, very low.
EEU Table 2
These are the risk factors so high-risk is prior VTE, patients with any combinations of one on the medium risk, which means age more than 75 (many of our patients), high BMI index, and we know that this is very important to cover. This was put up by Gordon Giat from McMaster University, and that I think, I don’t know how much in North America but does lead a lot of the VTE prophylaxis protocols in Europe at least.
VTE and radical cystectomy (RC)
VTE and radical cystectomy. The majority of patients that undergo radical cystectomy fall either into moderate which is about 3 to 6% event or high-risk VTE, which is about 11% as we saw according to the American college of Chest Physicians clinical practice guidelines. The risk of significant bleeding from VTE prophylaxis in this patient population is very low.
What is the optimal duration of pharmacologic VTE prophylaxis after RC?
What is the optimal duration for pharmacologic VTE prophylaxis after radical cystectomy and we all realize that we have to do that.
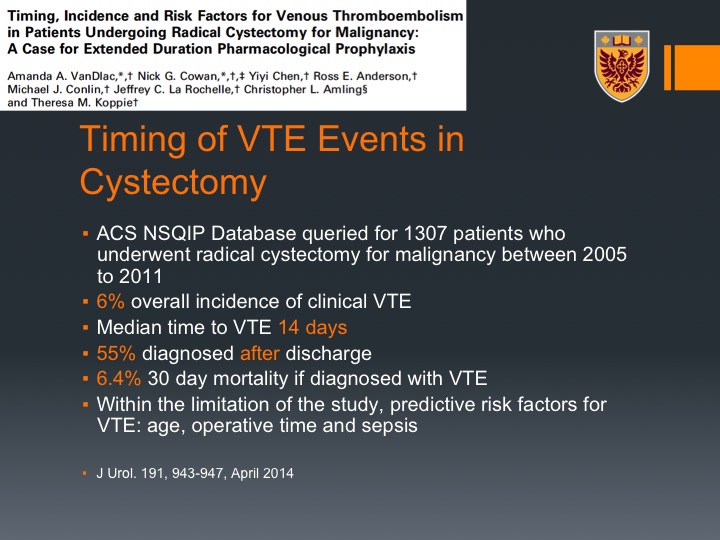
Timing of VTE Events in Cystectomy
This is data from Theresa Koppie who looked actually at the NSQIP data particularly for radical cystectomy so she had about 1300 patients who underwent radical cystectomy. She found again 6% overall incidence of VTE, the same percentage we saw overall. Median time to VTE was in 14 days, and in fact 55% or the majority of VTEs were detected after discharge and the mortality if you do have VTE or if you did have VTE was very high, was 6.4%, 30-day mortality is if you were diagnosed with VTE. This is just a graphic presentation for that, so message number one most or majority of VTEs post radical cystectomy occur actually after discharge, okay when the patient is not any more under our care.
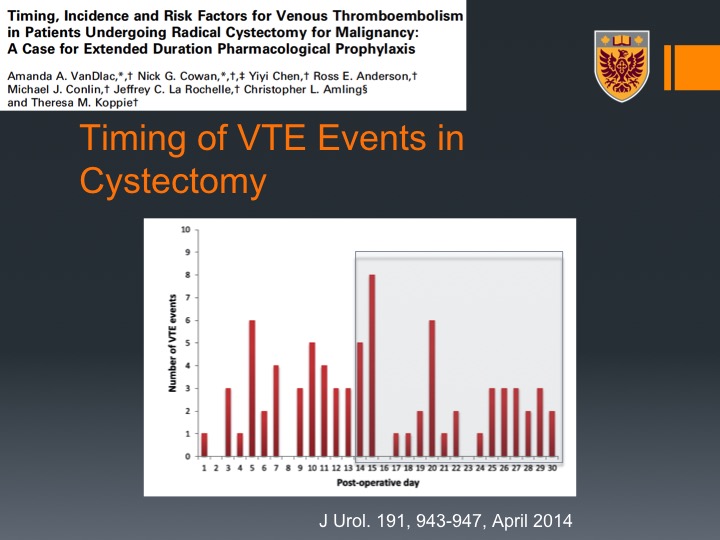
Timing of VTE Events in Cystectomy
This is just a graphic presentation for that, so message number one most or majority of VTEs post radical cystectomy occur actually after discharge, okay when the patient is not any more under our care.
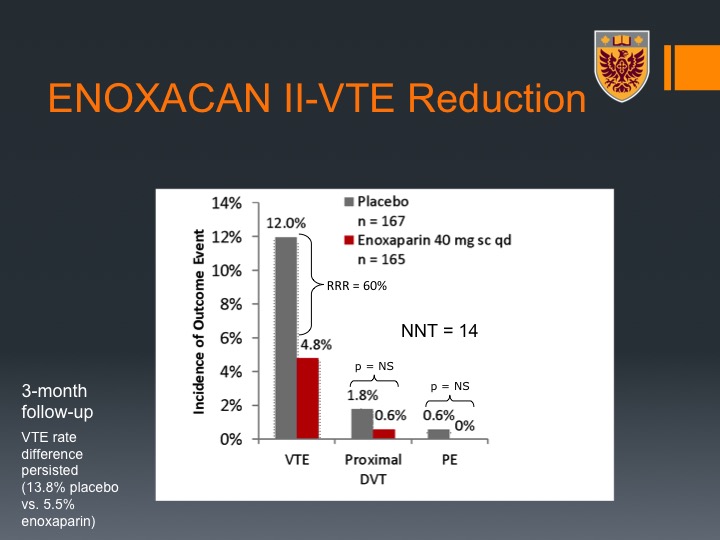
ENOXACAN II
The ENOXACAN II trial was a pivotal trial that addressed the question of duration of VTE prophylaxis in patients who undergo abdominal or pelvic surgeries for cancer, and this was a double-blind, randomized, multi-center trial that was done in a population that were planned to have open surgery for abdominal or pelvic malignancy not urologic specific, although about 10% of them were urological patients. They were divided between enoxaparin, which is low molecular weight heparin, 40 mg subcutan daily for about 28 days there or just at the time of the hospitalization, and the primary endpoint was actually radiographic, which is a bit of a caveat of this study.
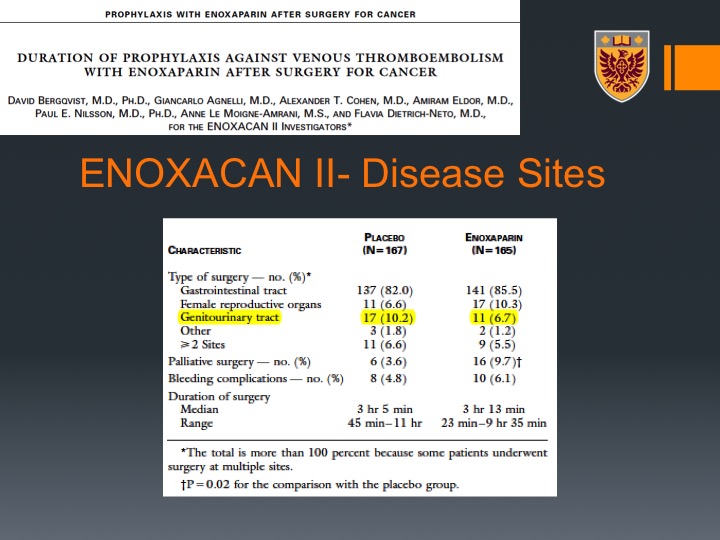
ENOXACAN II-Disease Sites
Nevertheless, one could see that it was significant therapeutic advantage for 28 days of VTE prophylaxis with enoxaparin that translated to a significant decrease in the events of VTEs along the 28 days.
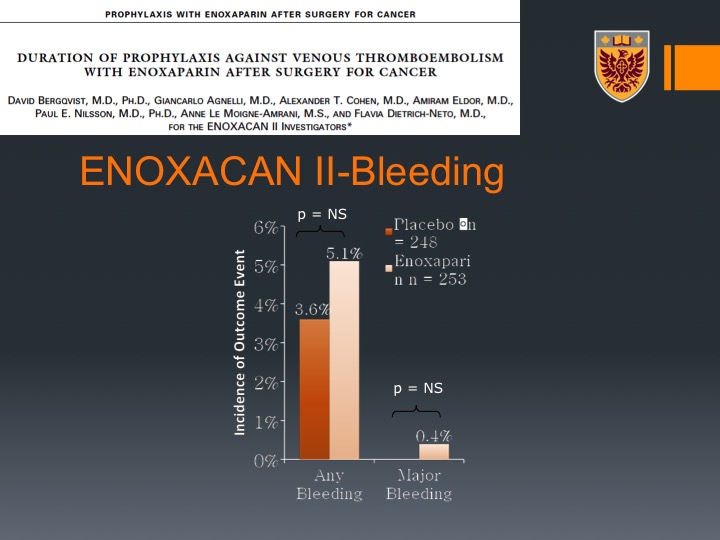
ENOXACAN II-Bleeding
It did not translate to higher or significant higher bleeding either any bleeding or major bleeding.
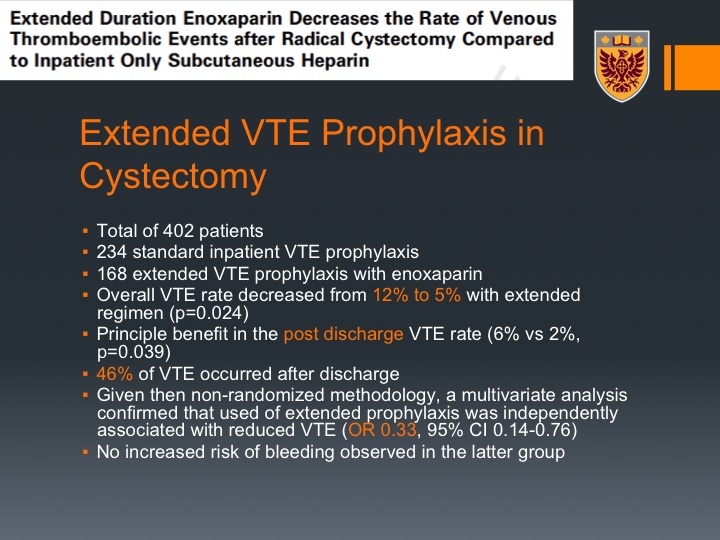
Extended Duration Enoxaparin Decreases the Rate of Venous Thromboembolic Events after Radical Cystectomy Compared to Inpatient Only Subcutaneous Heparin
This turned to be kind of a guideline for or recommendation for extended prophylaxis for abdominal pelvic surgery, cancer surgery, but it hasn’t been percolated into the urology practice, and then came this paper by Gary Steinberg from University of Chicago, and they actually looked back at their series, they adopted the 28 days of prophylaxis in January 2013, so they looked at the event rate before that and after that.
Extended VTE Prophylaxis in Cystectomy
And what they could show that in a total of 400 patients they could see that the overall VTE event decreased from 12% to 5% with the extended prophylaxis regimen, and the principal benefit was actually post discharge VTE rates from 6% to 2% which was clinically significant, and again about half of their patients had their VTE event after discharge, so this is very, very important to recall for the practicing clinician here. There was no increased risk of bleeding in the extended prophylaxis.
In patients with bladder cancer who undergo radical cystectomy
So patients with bladder cancer who undergo radical cystectomy VTE occurs with significant frequency. It’s about 6% if you look at many series, some of them quote more than that. VTE is associated with morbidity, cost, and mortality, and can be favorably modified through employment of extended low molecular weight prophylaxis for 28 days.
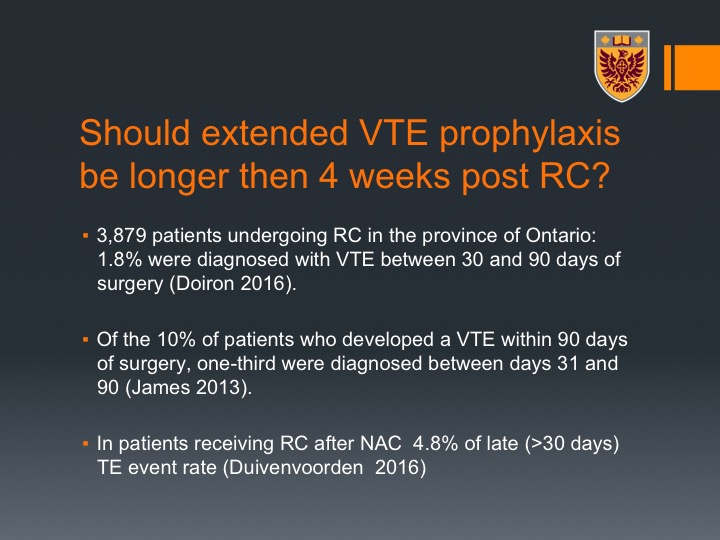
Should extended VTE prophylaxis be longer than 4 weeks post RC?
The question is should the extended VTE prophylaxis be longer than four weeks post radical cystectomy? There is actually data, interesting data from Ontario that about 1.8% of VTE were diagnosed even after 30 days, between 30 and 90 days from surgery and 10% of the patients who developed VTE within 90 days of surgery one-third were diagnosed between day 31 and 90. What we showed in our study on patients who undergo neoadjuvant chemotherapy followed by radical cystectomy was about 5% rate of late thromboembolic events, so more than 30 days. So even though we cover, and the guidelines go for 28 days, this is the ACP guidelines, still after 30 days we could see thromboembolic events.
Urologic Oncology
Now, we had an interest shifting into the neoadjuvant in seeing how the effect of Cisplatin-based neoadjuvant chemotherapy effects the occurrence of thromboembolic event, so we first looked at our institution 202 patients.
Main findings:
And we did show that there was enormous amount of patients about 19% who had VTE either at the time of the neoadjuvant chemotherapy or after the surgery. And this was compared to about 5.6% of patients went up front to radical cystectomy obviously matched by tumor characteristics. So we were quite puzzled by that.
Oncology Adrenal/Renal/Upper Tract/Bladder
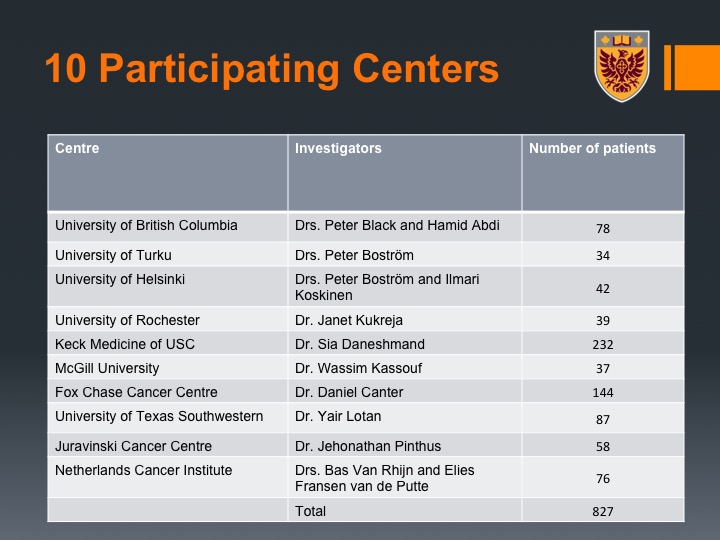
We embarked and led international group, Peter Black here contributed much to that as well, and we looked at multiple institutions to see if that is indeed the case.
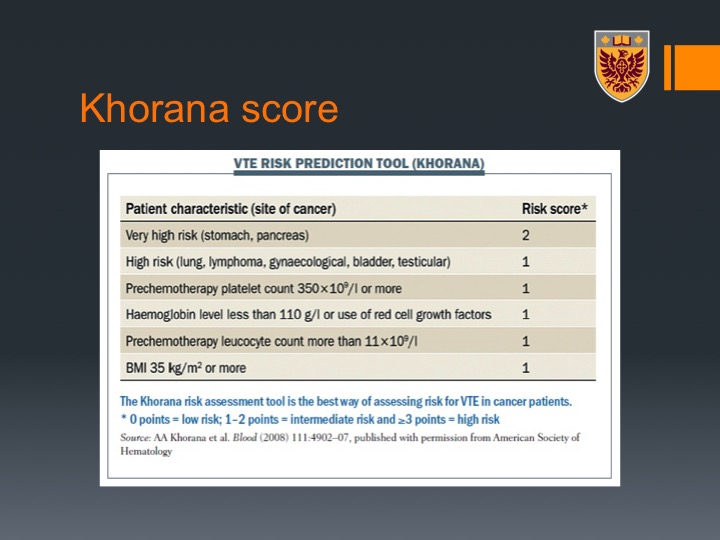
Khorana score
More than 800 patients we did look also at the Khorana Score. This is a score that classified patients who undergo chemotherapy to the risk of thromboembolic events and that relates mainly to the site of malignancy with bladder being high risk and getting one-point score and BMI as well as some CBC variant and I don’t have time to go over that. I will skip that for the interesting of time.
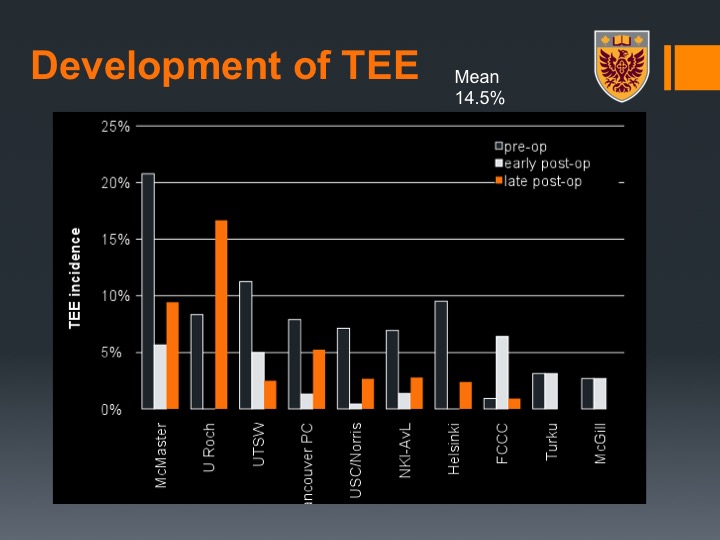
Development of TEE
What we could show was again across the study there was about a 14% rate of VTE, so not that far from our 19%. Here we marked by the center.
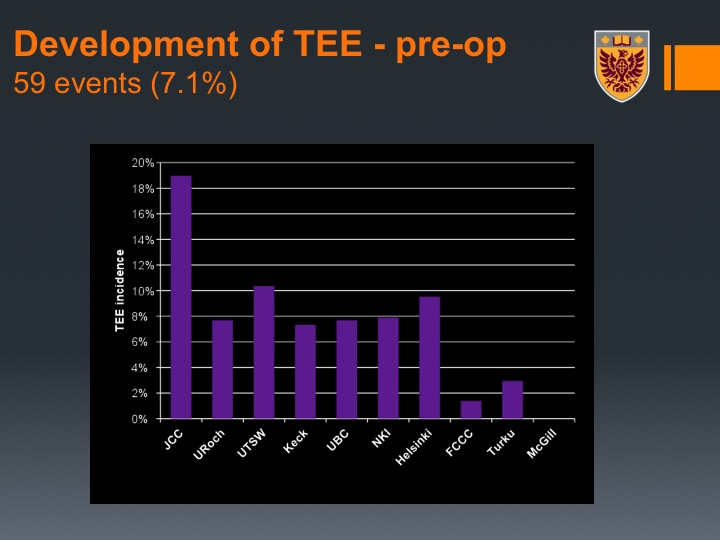
Development of TEE – pre-op
Interestingly enough pre-operative VTE occurred in about 7% of the patients so that is during the time of their neoadjuvant chemotherapy.
Post NAC imaging practice
What you could see here is actually there was a variation between the institutions, and we actually related that or we hypothesized this relates to the post-neoadjuvant chemotherapy imaging practice because not all centers at that time at least did CT of the chest. They did CT abdomen and pelvis but not CT of the chest.
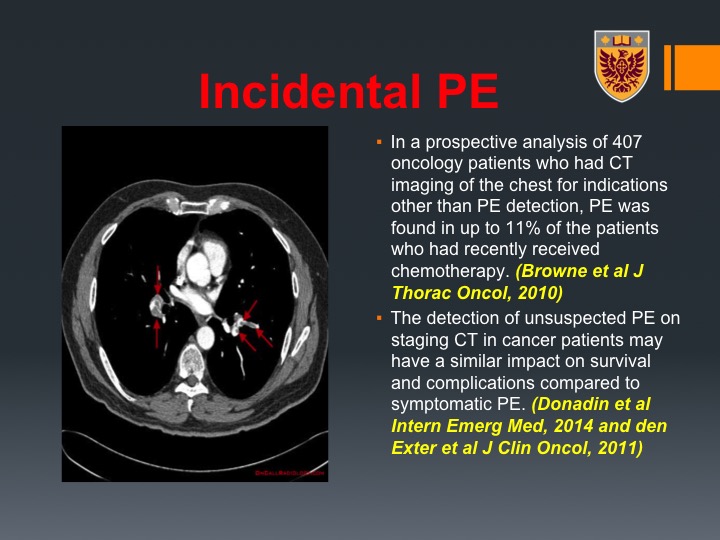
Incidental PE
This is very important. I hope you will capture this message because I think it is also very, very important. So we could find incidental PEs in staging CT that was done after the neoadjuvant chemotherapy just before the surgery. So you may say it’s incidental, patients are asymptomatic, but the literature, the thrombo literature, would suggest that these incidental PEs, which can be detected up to 11% of patients who undergo chemotherapy and chest imaging after that have the same impact on survival and complications compared to symptomatic VTE. So those are very important and I think including a CT of the chest not only would help you in terms of staging, but I think if you detect these incidental PEs, you do a service to the patient.
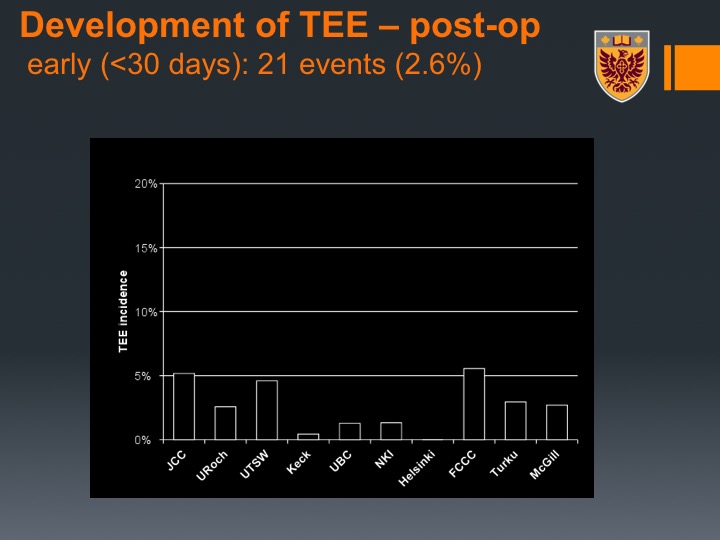
Development of TEE – post-op
So you can we divided again, so this was developing of TEE or thromboembolic events early, so this was about 2.6% in this patient population.
Development of TEE – post-op Continued
And late about 5%, so that potentially suggests, and we didn’t continue on with studies on that, that you may need to give for patients who undergo neoadjuvant chemotherapy followed by radical cystectomy even a longer period of VTE prophylaxis.
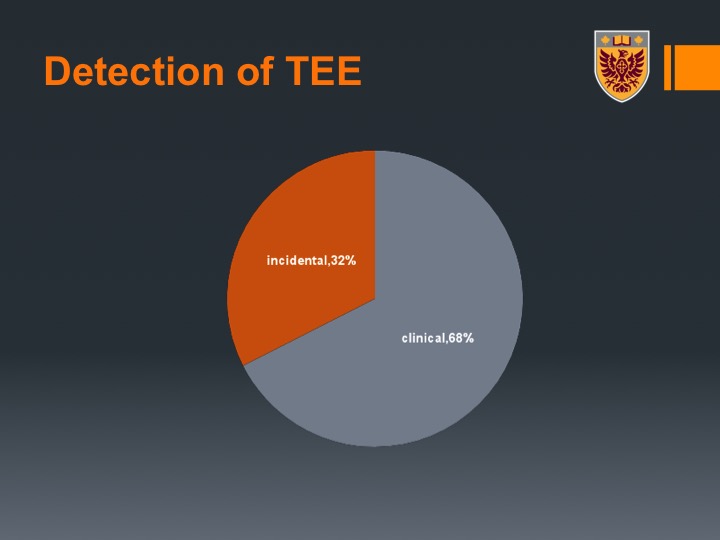
Type of TEE
Types of VTEs, were divided between DVTs and PEs, although as I mentioned there were arterial events. Speaking to people, some people had not in this series but speaking to people after that when we presented the data had for example mesenteric emboli or even a limb emboli.
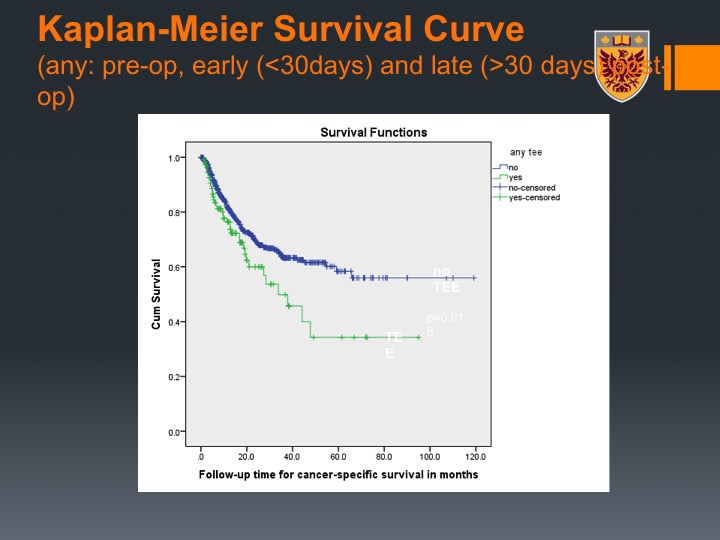
Kaplan-Meier Survival Curve
Interestingly enough, it seemed to be separating the curve of survival, so if you did have VTE event, you had worse survival, and that may correspond with the effect that tumors that are thrombogenic and secrete tissue factors and heparinase that are also associated with degradation of the extracellular matrix and developing lymph metastasis share the same profile
Conclusions
So in conclusion here, thromboembolic events were common, very common in bladder cancer patients who underwent neoadjuvant cisplatin-based chemotherapy. The majority and this is another lesson to recall here, occurred before the radical cystectomy so about 58% of the VTEs in this patient population occurs even before the cystectomy, we demonstrated that thromboembolic event is independent predictor for progression-free survival.
Gopalrkishna
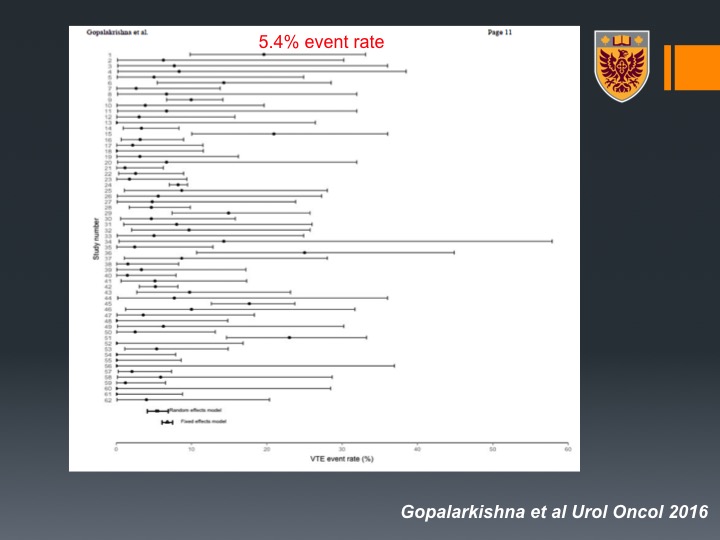
That’s about that. So now how about patients that are undergo chemotherapy for metastatic disease. So not in the neoadjuvant setting? Well there was this excellent systematic review by the group of Brant Inman. And this was systematic review and meta analysis of 62 studies, about 5,000 patients and in this patient population all around the world there were about 373 VTE events.
5.4% event rate
The rate is about 4.5% event rate on patients that are undergoing chemotherapy for bladder cancer. Now, as you can see there is a lot of heterogeneity. This is very interesting because when they looked at the origin of the studies, they could see that North American studies or American population had about 7% VTE events compared to Western non-North American with about 3.2% and less than that in non-Western countries.
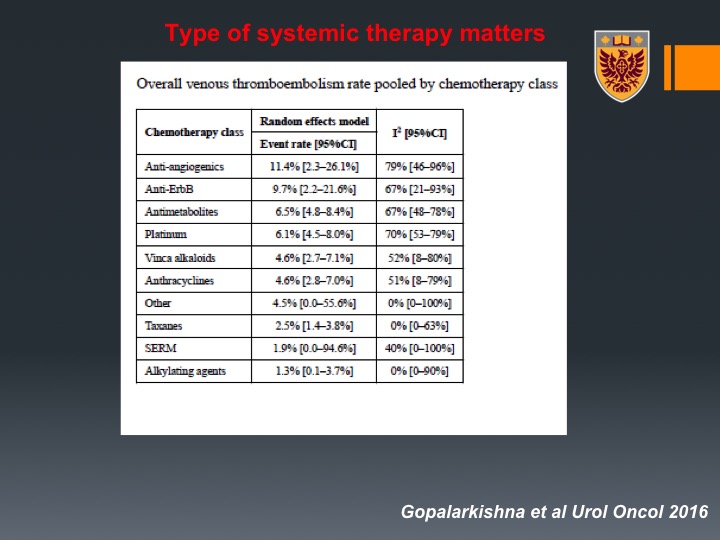
Type of systemic therapy matters
So that may relate to different practices in VTE prophylaxis, different types of imaging or different patient characteristics. Interestingly enough, they looked at all types of chemotherapy that were delivered to patients and one may want to recall that anti-androgenic such as bevacizumab had up to 20% events rate, very, very important.
Prior radiation increase the incidence of VTE
Interestingly enough MVAC had about 5% where gem/cis has about 6%. Another thing that was very interesting, the prior radiation increased the risk of VTE, so for example if you have bladder preservation and then you get chemotherapy, you still have high risk of VTE despite the fact that you didn’t undergo radical cystectomy.
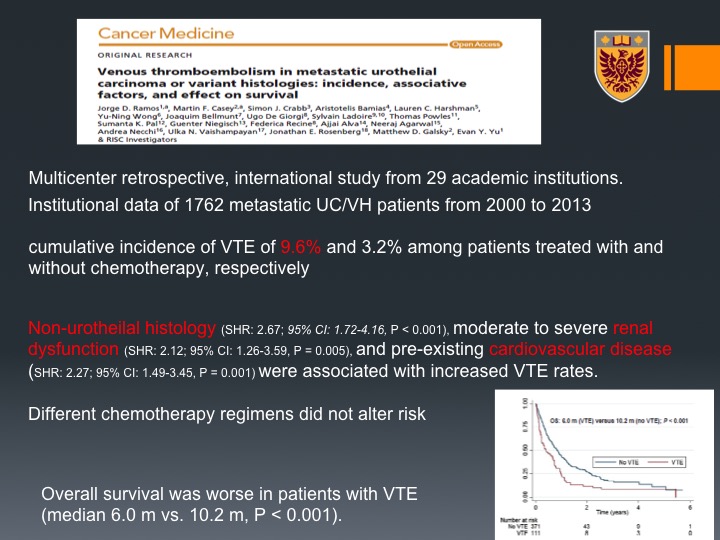
Cancer Medicine
Lastly, another I think interesting paper this is a multi-center retrospective international study of about 29 academic institutions and they could show that there is community of incidence of VTE of about 10% in patients who are treated with chemotherapy and the risk factors that they could identify were non-urothelial histology so variant histology if you will, renal dysfunction, cardiovascular disease.
Should we apply TEE Prevention During Chemotherapy in bladder cancer patients?
So this brings me to the end of the talk, which I would like to ask with you should we apply thromboembolic prevention during chemotherapy in bladder cancer patients? The risk is so high, should we do that? So essentially there are no trials that asked this question and tried to answer it in the bladder cancer population. But there was a trial the SAVE-ONCO trial, which was a randomized double-blind clinical trial of about 3200 patients with locally advanced or metastatic solid organ malignancy so not necessarily bladder cancer and they could show that there was a 64% reduction in the incidence of symptomatic VTEs when they used oral anticoagulants, semuloparin. So that was very, very interesting. However, the problem was that the incidence of VTEs in the placebo group was about 3.4%, and the reduction, the absolute risk reduction was about 2% only, and based on that the FDA did not approve this prevention strategy in ambulatory cancer patients with solid tumors that undergo chemotherapy.
Current Guidelines
The current guidelines by ACCP, the American College of Chest Physicians and ASCO recommend against routine prophylaxis in ambulatory patients with cancer receiving chemotherapy with the exception of those with high risk factors, for example, previous VTE, immobilization, hormonal therapy, this angiogenesis inhibitor therapy and thalidomide.
Why do I still think that we need to study the effect of thromboprophylaxis in chemotherapy treated bladder cancer patients?
I would leave you with that that I still think there is a rationale to run as preventive study in patients with bladder cancer, and the reason is that if you look at bladder cancer the risk is very, very high, way higher than the 3.4% that was the baseline risk of the patients in the SAVE-ONCO trial, so I do argue that potentially if we do apply prevention strategies particularly in patients who undergo neoadjuvant chemotherapy where they have a basis of about 14 to 15% chance of having VTE that we’ll see a higher absolute risk reduction. Perhaps that would be towards the benefit of our patients. Thank you.
ABOUT THE AUTHOR
Dr. Pinthus is an Associate Professor in the Department of Surgery’s Division of Urology at McMaster University in Hamilton, Ontario. He is a surgical oncologist-urologist who completed his MD at the Hebrew University in Jerusalem, Israel, and his urology training at the Sheba Medical Centre at Tel Aviv University in Israel. He then completed 2.5 years of an SUO-accredited Fellowship in Uro-Oncology at the University of Toronto. Dr. Pinthus earned his PhD at the Weizmann Institute of Science in Rehovot, Israel, working on gene immunotherapy of prostate cancer.
He is heading a basic and translational research laboratory at McMaster University and his research interests relate to how patients’ individual host factors and metabolism affect prostate cancer growth and response to therapy. Dr. Pinthus has published more than 80 peer-reviewed papers and several book chapters. He is a member of the editorial board of European Urology, Prostate Cancer, and Prostatic Disease. Dr. Pinthus is the principal investigator on many distinguished research grants, including a $3.5 million Movember-Prostate Cancer Canada grant titled “Role of androgen deprivation therapy in cardiovascular disease - a longitudinal prostate cancer study (RADICAL PC).” This study is intended to prospectively address the potential link between prostate cancer in general and androgen deprivation therapy (ADT) in particular and an increased risk of cardiovascular disease.