Daniel P. Petrylak, MD, presented “New Targets for the Treatment of Urothelial Cancer” at the 2nd Annual International Bladder Cancer Update on January 27, 2018 in Beaver Creek, Colorado
How to cite: Petrylak, Daniel P. “New Targets for the Treatment of Urothelial Cancer” January 27, 2018. Accessed Nov 2024. https://New-Targets-for-the-Treatment-of-Urothelial-Cancer/
Summary:
Daniel P. Petrylak, MD, discusses recent advances and clinical trials for targeted checkpoint inhibition therapy in the setting of metastatic urothelial carcinoma.
(Twitter Question) Reveal the Answer to Audience Response Question #1
True or False- PDL1 expression is a useful marker of response to checkpoint inhibitors?
- A. True
- B. False
Answer Explanation:
The answer is False. However, you could argue to the contrary depending upon which assay you use and which treatment.
Reveal the Answer to Audience Response Question #2
Since median progression-free survival is not improved in patients treated with checkpoint inhibition therapy, what is the best endpoint?
- A. Objective response rate
- B. Overall survival
- C. One-year survival
- D. Two-year survival
New Targets for the Treatment of Urothelial Cancer – Transcript
Click on slide to expand
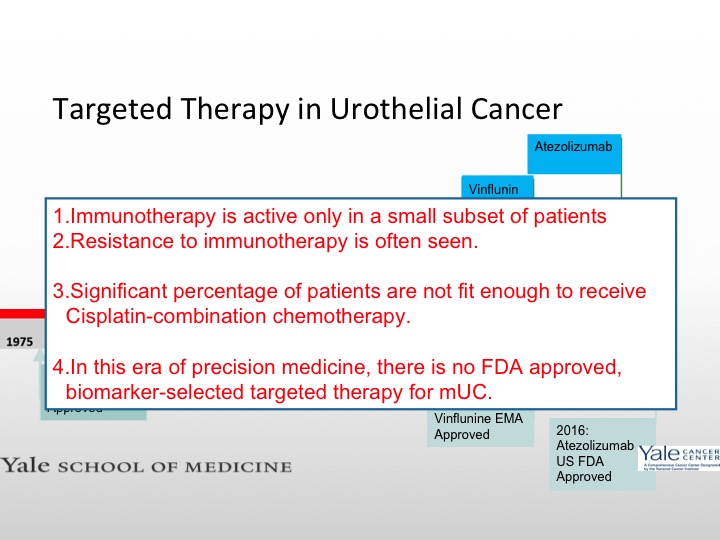
Targeted Therapy in Urothelial Cancer
As we mentioned earlier immunotherapy is active in a quarter of patients with metastatic urothelial carcinoma. We often see resistance although I think the durability of response is probably the best with these treatments that we’ve seen with any agent to date, and there are a significant percentage of patients who are not fit enough to receive Cisplatin-based therapy, so again, we are looking for other targets. Right now we don’t have any FDA approved precision agent for bladder cancer, but we hope to see that in the near future.
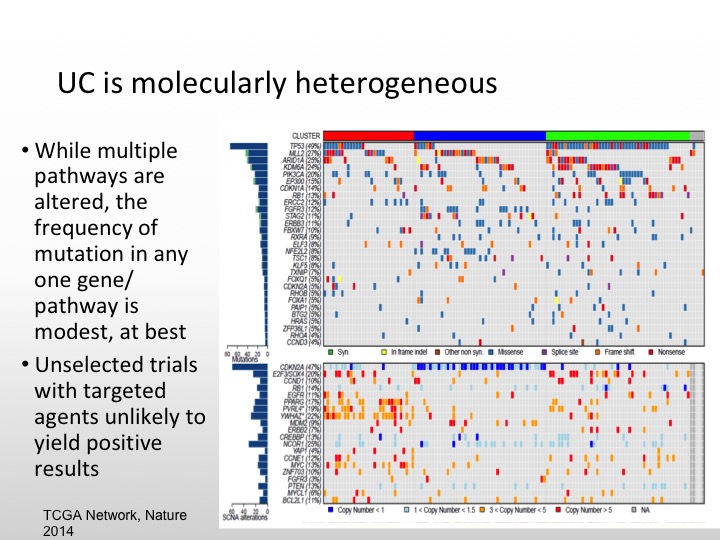
UC is Molecularly Heterogeneous
As we saw from Dan’s talk, urothelial carcinoma is molecularly heterogeneous. There are multiple pathways that can be altered, but there is not one specific gene pathway like we see with CML in his last example. That is the best one to target, so unselected trials in the past have caused us problems. We see agents that may have more activity if we select out for different individual markers so it is unlikely that I think the small study is going to show positive results unless we enrich our patients for particular markers.
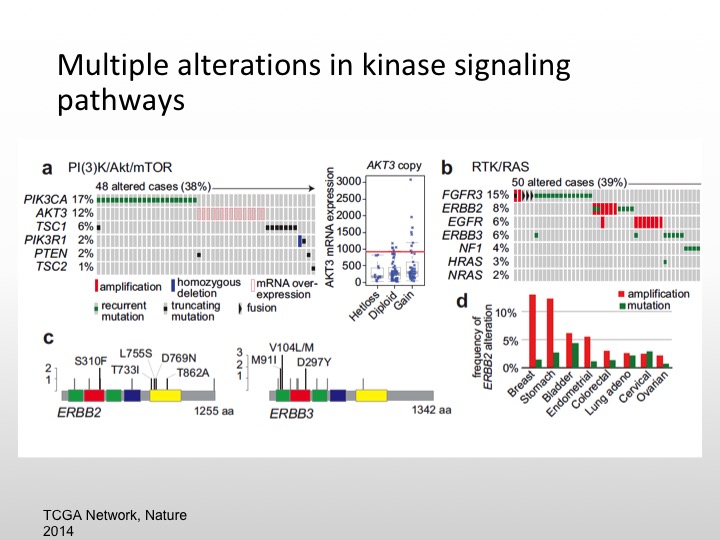
Multiple Alterations in Kinase Signaling Pathways
So we know there are multiple kinase pathways that are expressed in urothelial carcinoma, namely the PI3 kinase or MTOR pathway. We also the FGFR3 pathway, retinoblastoma gene is deleted, P53 is mutated, FGF is also mutated, same thing with EGF, about 20% of specimens will express HER-2/nue. So again we have a variety of different areas that we can pursue and again if these are low-level expressions of particular markers we have to enrich and we may not see any particular results.
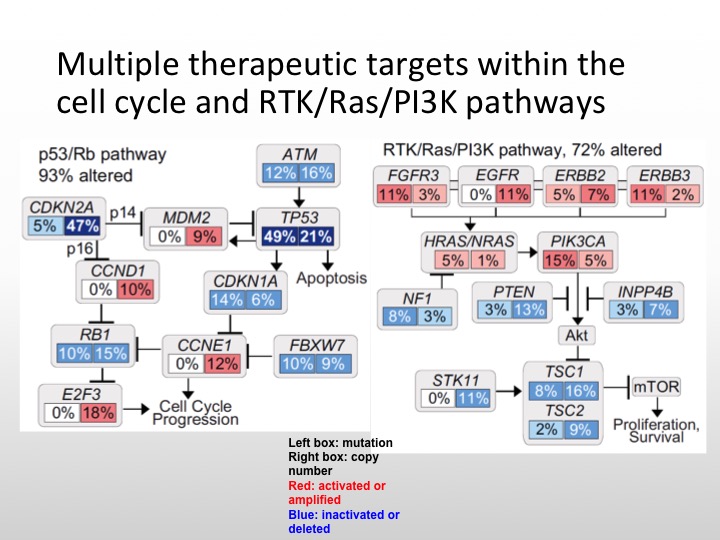
Multiple Therapeutic Targets within the Cell Cycle and RTK/Ras/P13K Pathways
So this is what I was saying before Ras/RAF kinase pathway, P53- all of these can be targeted. Unfortunately, even though P53 is expressed or mutations are seen in a large portion of bladder cancers, we do not have a specific agent, this is not a drug over target at this particular point, so the actual mutation itself is not targetable.
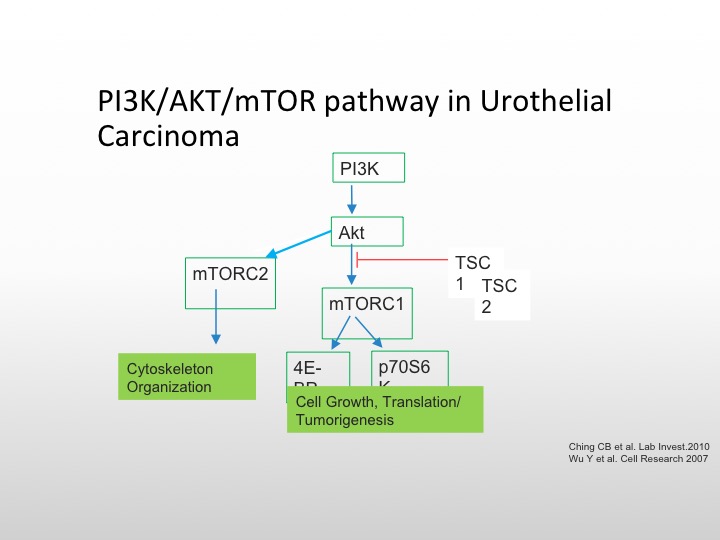
P13K/AKT/mTOR Pathway in Urothelial Carcinoma
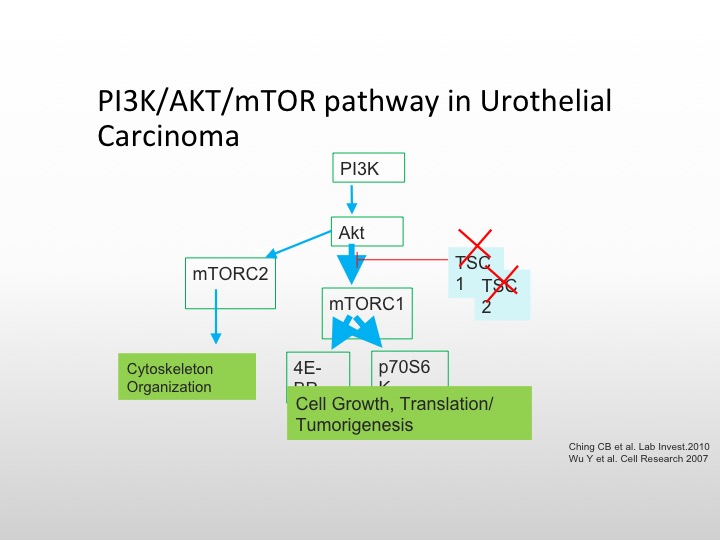
I’m going to focus first on the mTOR/P13K pathway, the TORC complex is important on the left for cytoskeletal organization, and the mTORC pathway, which goes to the 4AF3A and the transcription complex is responsible for cell growth and tumorigenesis, and there are again as we see here, several different pathways that we can affect the conversion of AKT the mTOR pathway, and again TSC1 and TSC2 mutations are also involved in this particular pathway.
P13K/AKT/mTOR Pathway in Urothelial Carcinoma
So you can knock those out and then cause unopposed cell proliferation and growth. Everolimus is known to affect the TORC complex, and we know that is approved for metastatic renal cell carcinoma, there have been studies in bladder cancer, but again, they have not been enriched to the level that we like to.
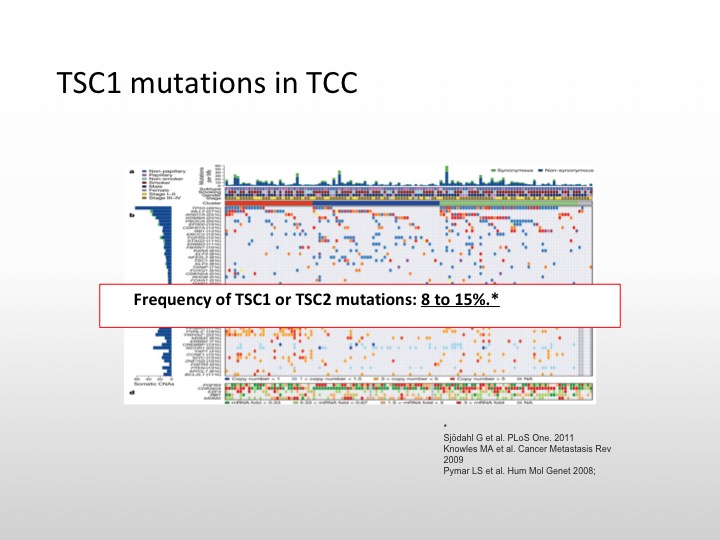
TSC1 mutations in TCC
So we do know that there are TSC1 mutations in transitional cell carcinoma. It occurs in about 8 to 10% of specimens that have been examined.
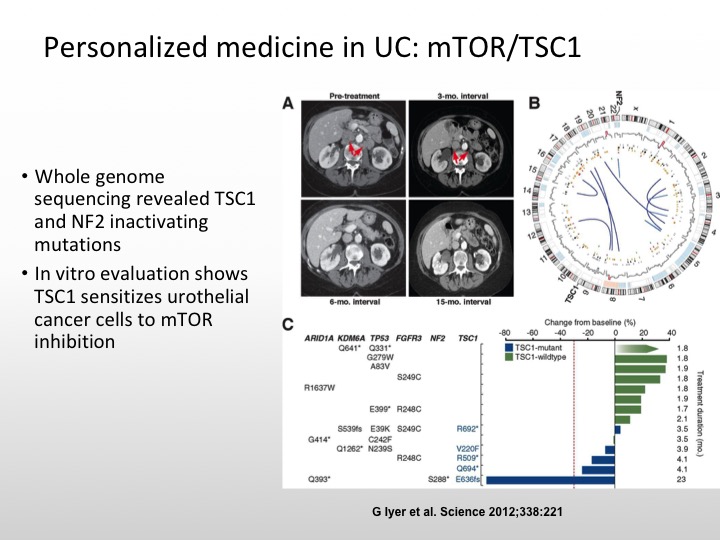
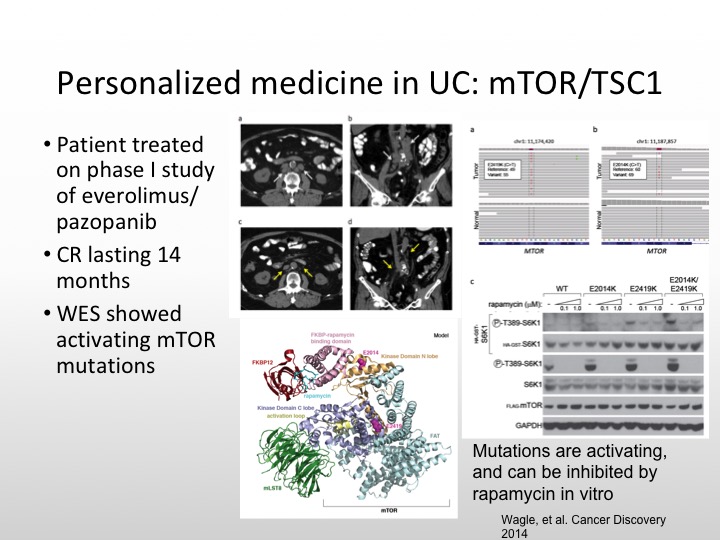
Personalized Medicine in UC: mTOR/TSC1
And the really important case I think that really got us to start thinking about this issue of molecularly targeted and personalized medicine in urothelial carcinoma was the patient who was treated on an Everolimus combined with pazopanib trial and this patient had whole genome sequencing, and this patient had a really dramatic response to this combination. And it was found that this patient had a TSC1 mutation and that is what was the explanation for this patient’s dramatic response.
Personalized Medicine in UC: mTOR/TSC1
And this patient had a complete response that lasted 14 months, and there were activating mutations of mTOR that were seen as well. So not all size fits one. We have to be sure that we have the right population that we’re studying.
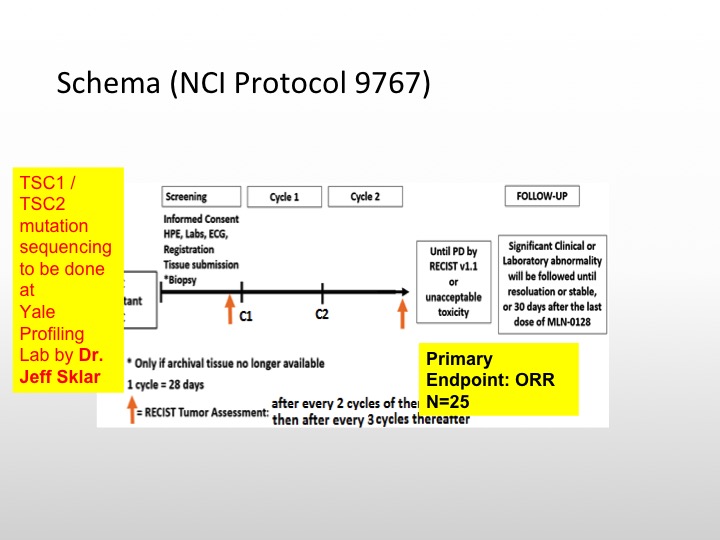
MLN-0128 (aka. TAK228): NCI Protocol 9767
At Yale, we are currently looking at a new compound. This is actually being done through the phase 2 cooperative group through the NCI, Joseph Kim, my junior attending is actually running this trial, and what we are looking at is an ATP competitive inhibitor of mTOR kinase that will basically go after the TORC1 TORC2 complex and this actually shows inhibition of TORC1 TORC2 at very, very, low levels, and this potentially has greater clinical activity to those drugs that are out there in the community like Everolimus and temsirolimus.
Schema (NCI Protocol 9767)
So we’re actually going to be doing TSC1 and TSC2 mutation sequencing on these patients. We’re going to select 25 patients for this particular study, and see if enriching these patients will lead to a better response rate or reasonable response rate with this particular TSC1/TSC2 inhibitor. So this is a targeted therapy trial that is selecting patients based upon TSC1 and TASC2.
Epidermal Growth Factor
The epidermal growth factor receptor family is expressed in probably about 70% of urothelial carcinoma specimens. The difference between EGF in lung cancer and bladder cancer is you don’t see mutations to the same level that you do in lung cancer it is a very, very rare event, and why is that important? Because those will predispose you to responses to some of the tyrosine kinase inhibitors that are very, very active in lung cancer. So we’re really not absolutely sure of the exact role of EGF, whether it’s differentiation factor or it’s a proliferation factor, and in fact, it may be dependent upon the molecular phenotype of the tumor that is examined.
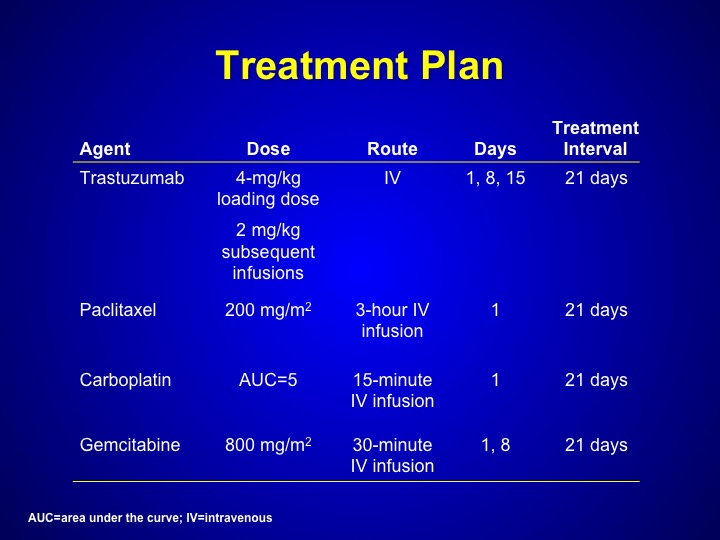
Treatment Plan
But Maha Hussain looked at trastuzumab, which is Herceptin. That’s a HER-2/neu inhibitor in patients who had any form of HER-2/neu expression a bladder cancer this could have been through serum. This could have been through tissue, any primary DNA analysis. And she treated these patients with a triplet combination of paclitaxel, carboplatin, gemcitabine along with trastuzumab.
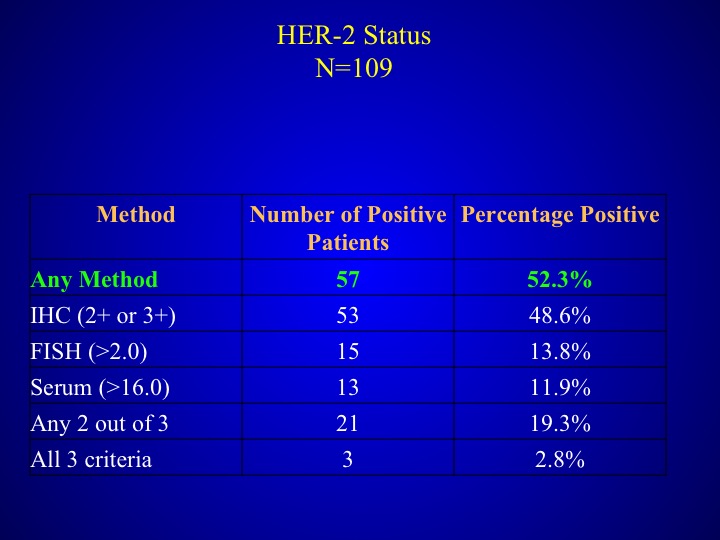
HER-2 Status
As I mentioned before any method was used, half of the patients had at least one method several of the other patients had 2.8%- had all three criteria.
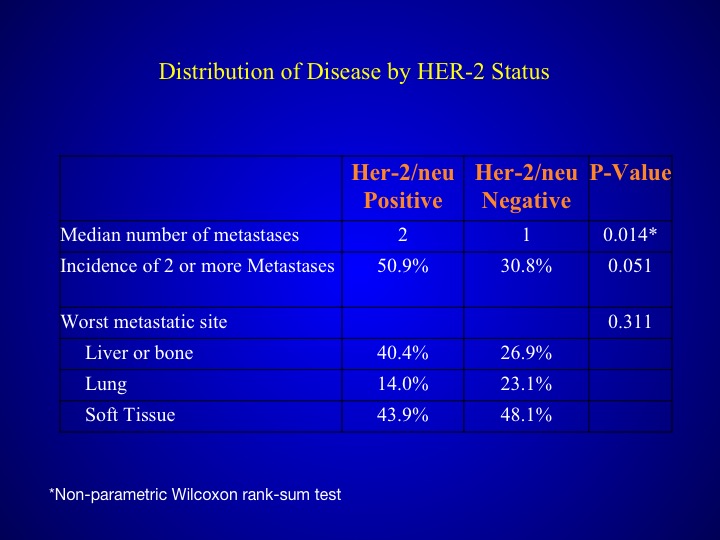
Distribution of Disease by HER-2 Status
When you look at those patients who had HER-2/neu expression, they tended to have a poorer prognosis, more number of metastases and also more visceral sites.
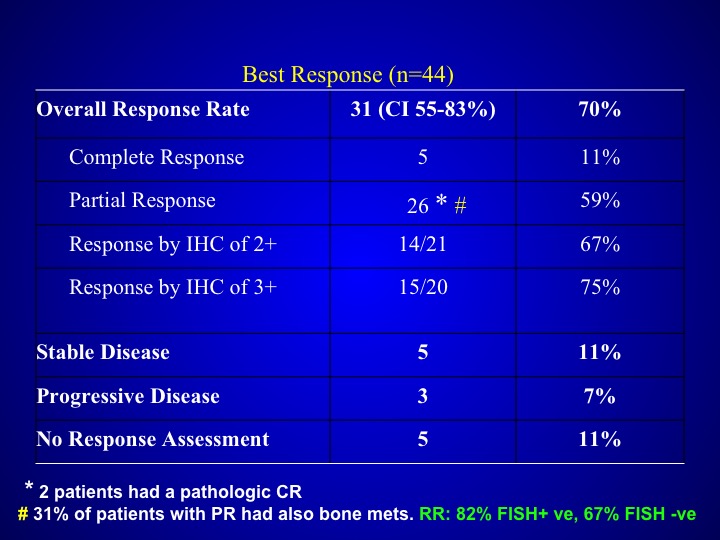
Best Response (n=44)
Response rates did not seem to be particularly different than what you saw with patients who were treated with that triplet regimen alone with out the trastuzumab and were not selected but if you look at the data carefully, these patients again seemed to have more aggressive disease.
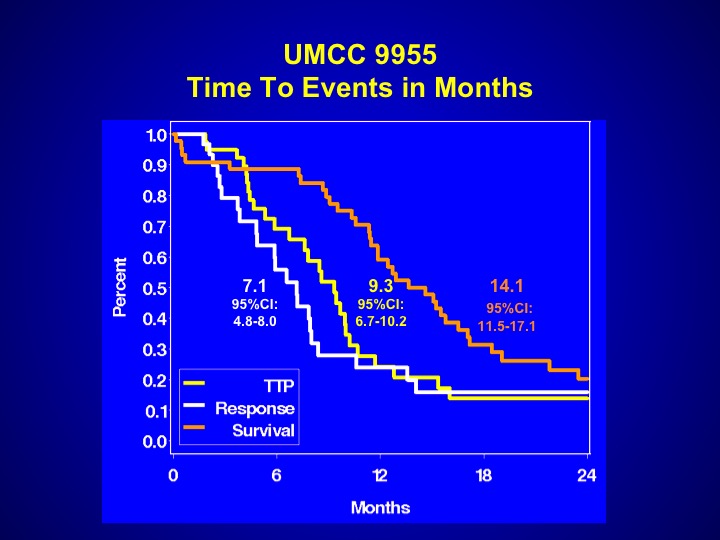
UMCC 9955
And perhaps that is the reason why the events that were seen with this particular trial were somewhat worse. The survival was 14.1 months, a little bit worse than what you expect with chemotherapy, but again it may be patient selection. This drug has never been evaluated properly in a randomized Phase 3 trial.
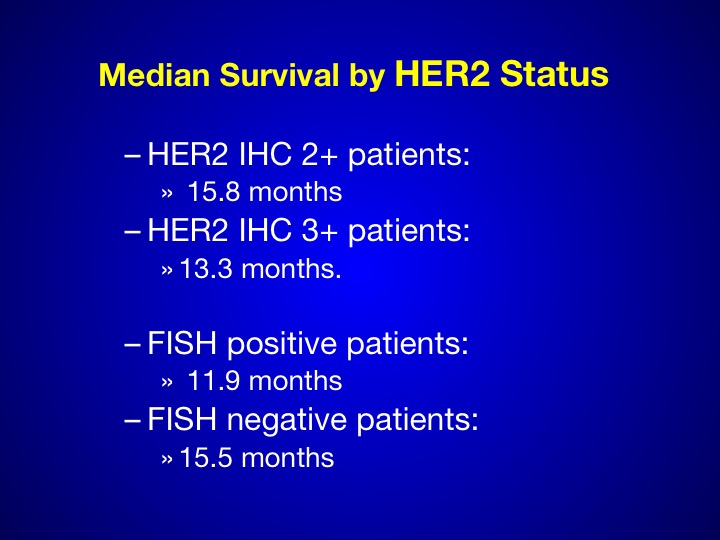
Median Survival by HER-2 Status
Again we see in the 3+ patients, a small number is hard to determine anywhere between 15 and 18 months.
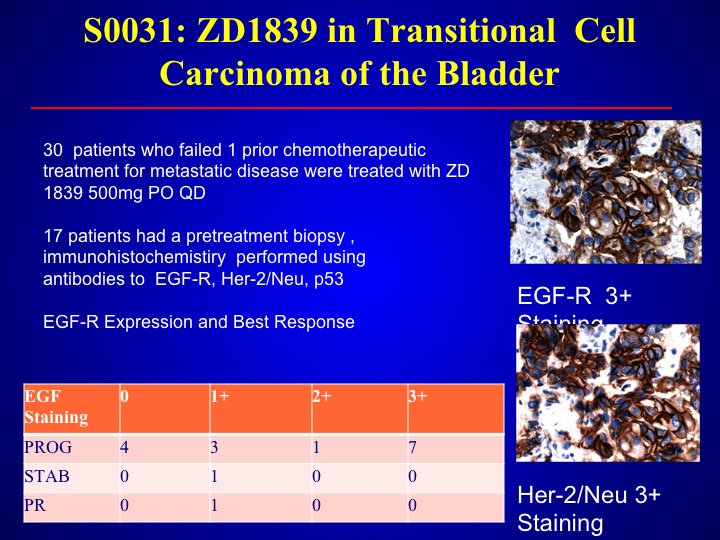
S0-031: ZD1839 in Transitional Cell Carcinoma of the Bladder
Same thing with the – – which is also an EGFR antagonist. We looked at this data in the southwest oncology group, found only one response out of 30 patient and again we didn’t select but we could not—this was before the era of looking for mutations, and larger trials found there were no mutations in bladder cancer with EVGFR.
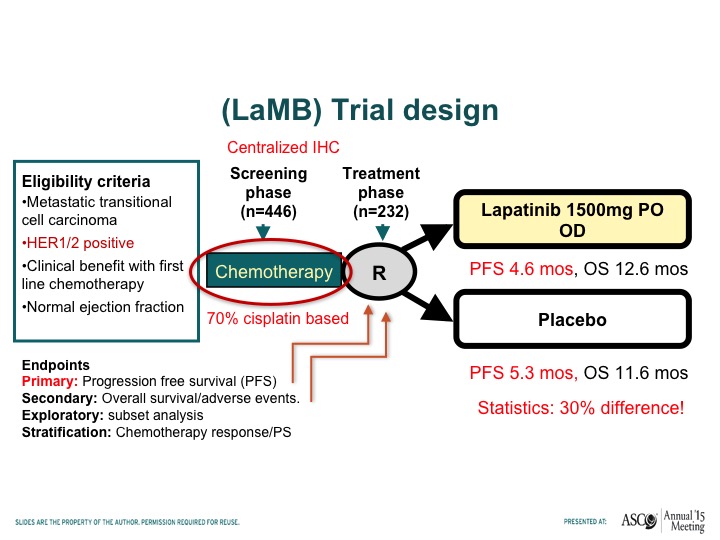
(LaMB) Trial Design
Tom – – has looked at lapatinib, which is another HER-2/neu tyrosine kinase inhibitor. Compare that to placebo in patients who had responded to chemotherapy this was a maintenance trial and this trial basically showed no difference in progression-free survival when patients receive lapatinib or placebo after responding to chemotherapy.
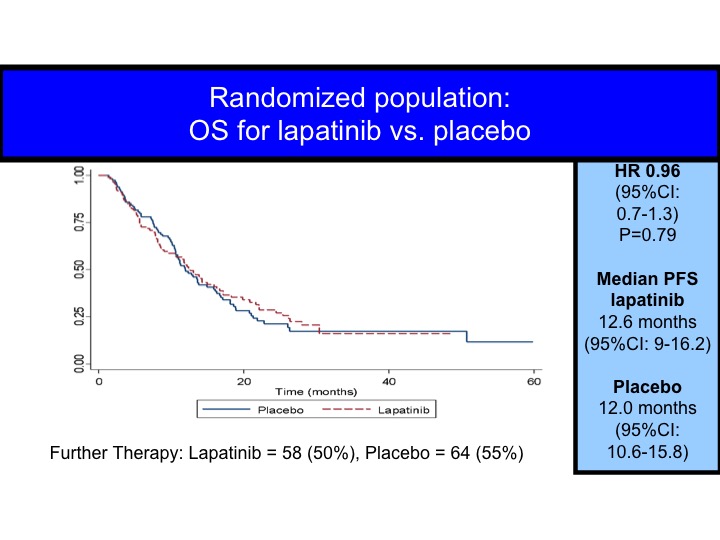
OS for Lapatinib vs Placebo
When you look at this in terms of all patients, you can superimpose the survival curves, but even for the HER-2/neu positives we don’t really see the difference.
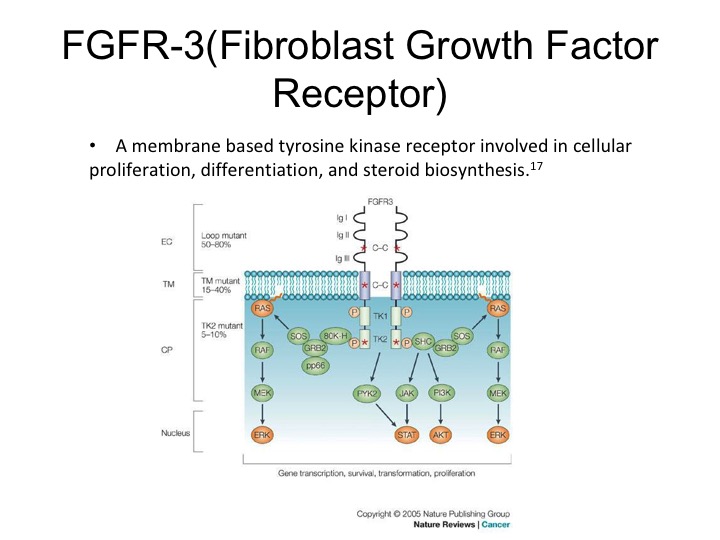
FGFR-3
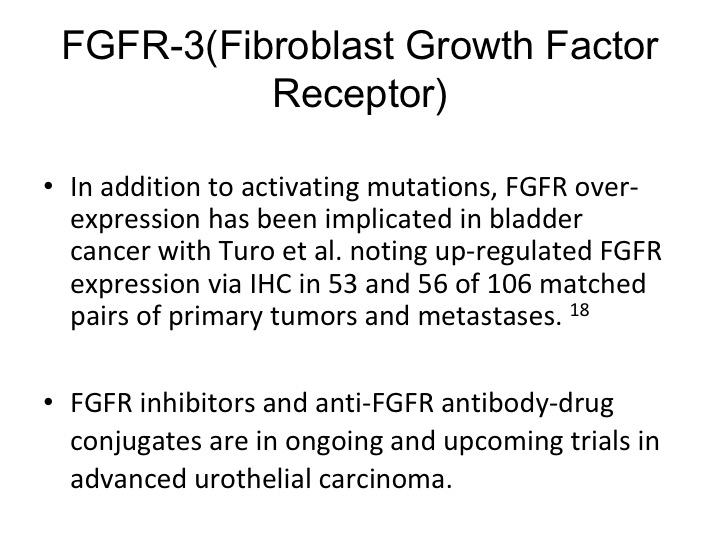
So this therapy or axis may or may not be active. We may not have the right drugs. Dan mentioned before that FGFR3 is expressed in urothelial carcinoma. It does seem to be more strongly expressed in non-muscle invasive disease. The muscle invasive disease but nonetheless it is a therapeutic target that can be exploited, there are specific FGR3 inhibitors that are now in clinical trials and these are showing response rates of about 20 to 30% in selected patients.
FGFR-3 (Fibroblast Growth Factor Receptor)
So they are activating mutations and there is also up regulation seen of the particular receptor.
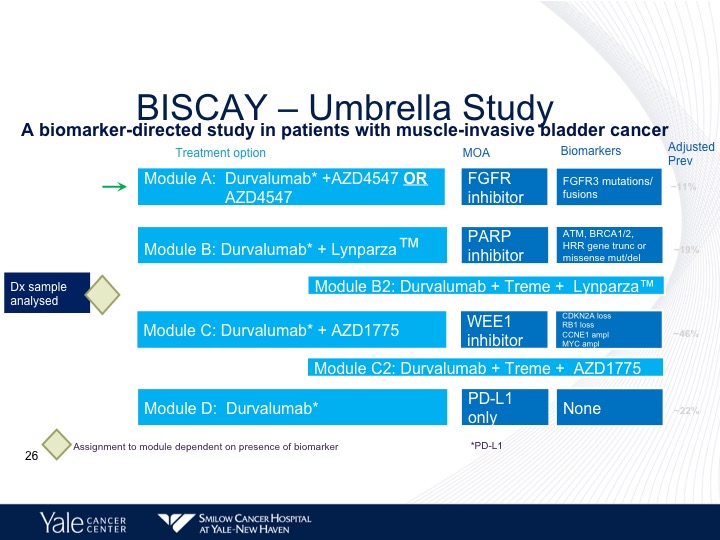
BISCAY—Umbrella Study
Now there are trials that are looking at combining some of these targeted agents along with immunotherapy. The BISCAY trial is looking at basically three different inhibitors, one is PARP inhibitor, Lynparza, the second, is an FGFR3 inhibitor. And the third is a WEE1 inhibitor, which is basically going after retinoblastoma, and this study is still ongoing. And we don’t have any results at this particular point.
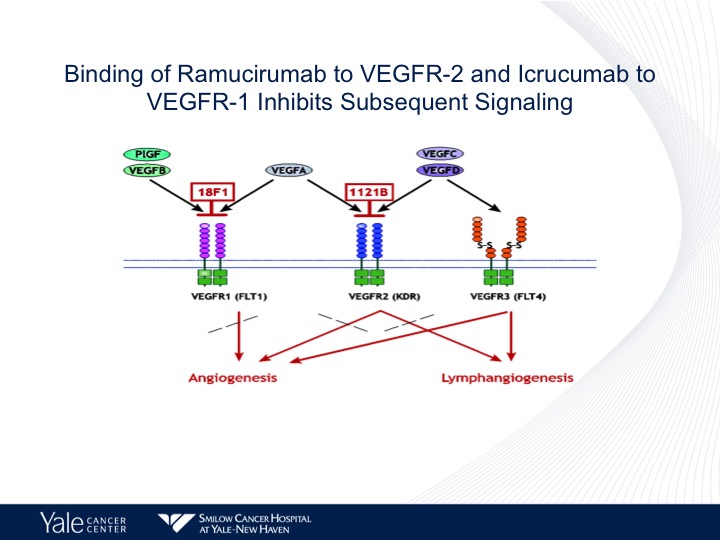
Binding of Ramucirumab to VEGF-2 and Icrucumab to VEGFR-1 Inhibits Subsequent Signaling
We have been focusing on the FEGFR-2 axis as we know there are several members of the VEGF family. There are several different drugs that are used and are FDA approved that target VEGF. Bevacizumab approved for lung cancer is predominantly targeted VEGFR-1. Ramucirumab predominantly targets VEGFR-2 and these have different effects on both lymphangiogenesis as well as angiogenesis in patients with urothelial cancer as well as other tumors.
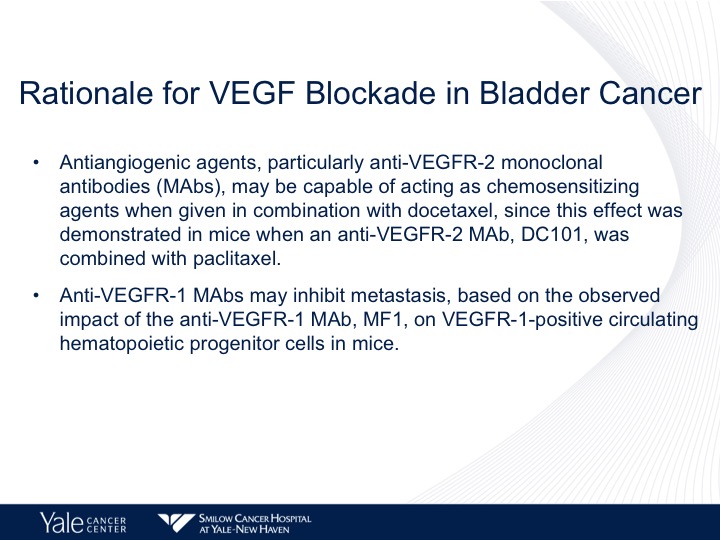
Rationale for VEGF Blockade in Bladder Cancer
In the laboratory it’s been shown that antibodies to VEGFR-2 combine with docetaxel chemotherapy can be chemosensitizing and also the VEGFR-1 pathway may inhibit metastatic disease and again also does have interaction with chemotherapy as well.
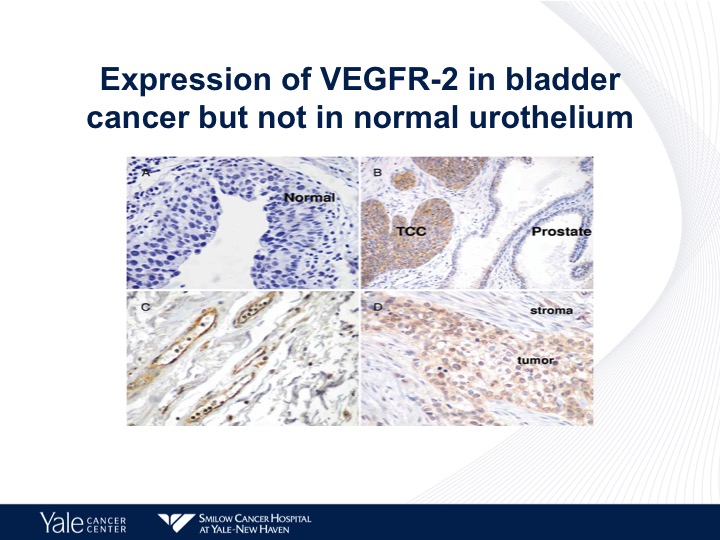
Expression of VEGFR-2 in Bladder Cancer but not in Normal Urothelium
This is an IHC chart looking at the different expressions of VEGF in human tissue specimens, as we can see on the lower right, urothelial carcinoma has a high level of expression of VEGFR-2.
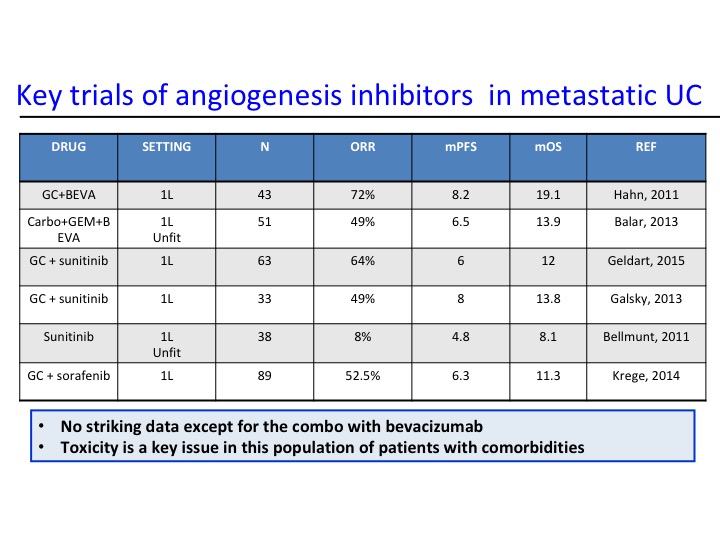
Key Trials of Angiogenesis Inhibitors in Metastatic UC
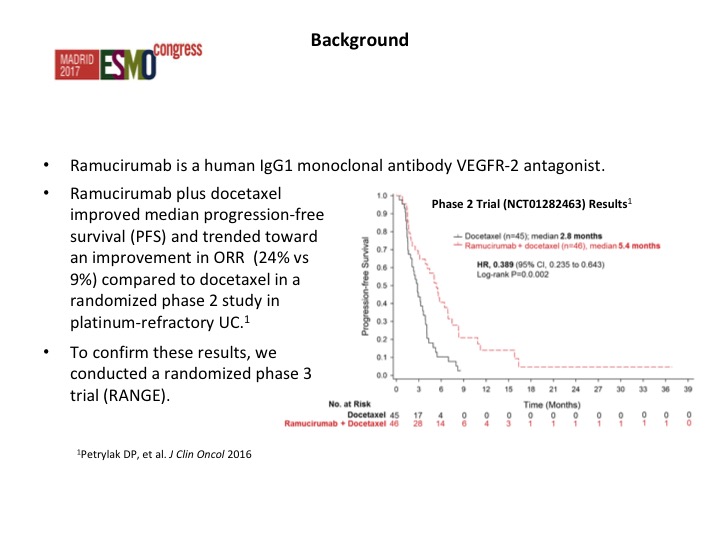
Now angiogenesis inhibition therapy has been a bit disappointing in urothelial carcinoma. In the past with Phase 2 trials there has really nothing that stood out as being particularly active, and of course we have toxicity, particularly hypertension that are seen with these particular agents. As we start looking at some of the combinations, we start seeing maybe a trend and at Yale we did a Phase 2 trial of docetaxel plus or minus ramucirumab and we saw about a doubling of the progression-free survival. That is the fourth line from the bottom and the second line from the bottom. This led to a larger Phase 3 trial that evaluated this.
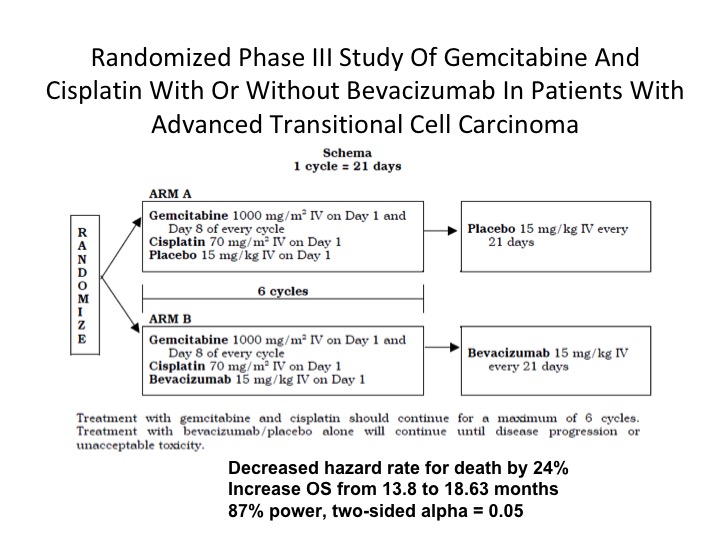
Randomized Phase III Study
There is still one phase 3 trial that is still outstanding as far as any data is concerned and this is one that is being performed in the intergroup mechanism. Gem/cis plus bevacizumab compared to gem/cis alone. It’s still not mature, and hopefully we will see something coming out this year on this study.
ESMO Congress
We went after the second line group because of our phase II experience and we presented this data, this was published in the Lancet back last September- it was presented at the ESMO meeting where we looked at the RANGE trial, which compared ram/docetaxel to docetaxel.
Background
These are patients who had prior chemotherapy, one prior chemotherapy for metastatic disease. Also they had to have failed that but they could have had checkpoint inhibition therapy, and in fact 10% of our patients on this study did receive prior checkpoint therapy.
RANGE Trial Design
Other key inclusion criteria, – – performance status of 01. We stratified this based upon geography. About 10% of our patients did have prior checkpoint, so this again was designed before the checkpoint era really was unleashed.
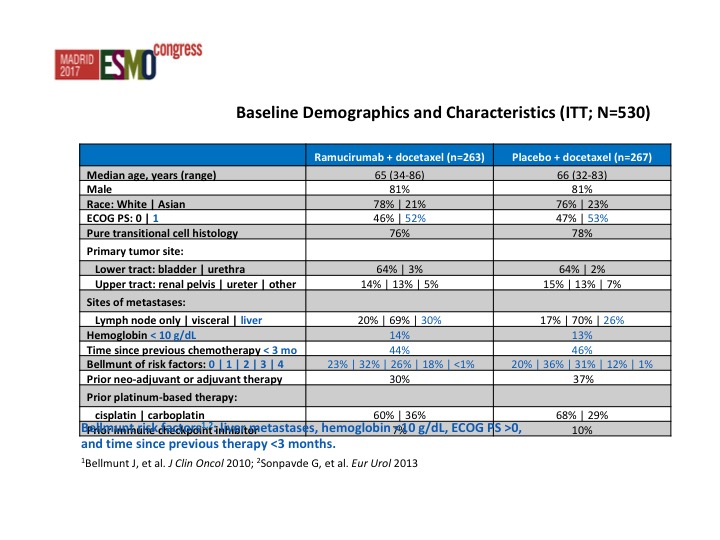
Baseline Demographics and Characteristics (ITT; N=530)
Very similar baseline demographics from both arms. This is a heavily or at least a poor prognosis group of patients and we start looking at the Belmont criteria, all patients at least 50% of patients had 2 or more poor prognostic features.
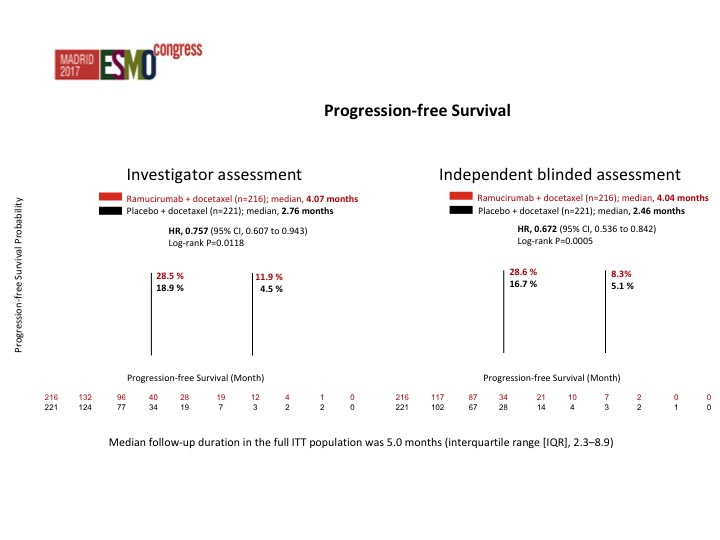
Progression-free Survival
This was both by investigator assessment as well as by an independent review. Actually the data from the independent review looked better than the investigator assessment but there was about a 25% reduction in the risk of progression which was significant in favor of RAM docetaxel. The survival data is not mature yet. I hope we will have an answer at some point this year as to whether there is an improvement in overall survival. That is going to be the key, but these are co-primary endpoints and I think the FDA depending on how close the survival data is, I don’t want to speculate, but they may still accept it even though the survival data may not be significant.
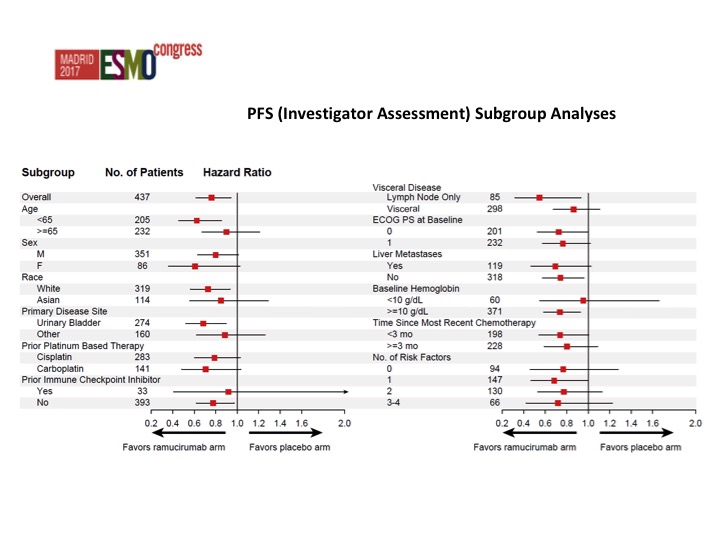
PFS (Investigator Assessment) Subgroup Analyses
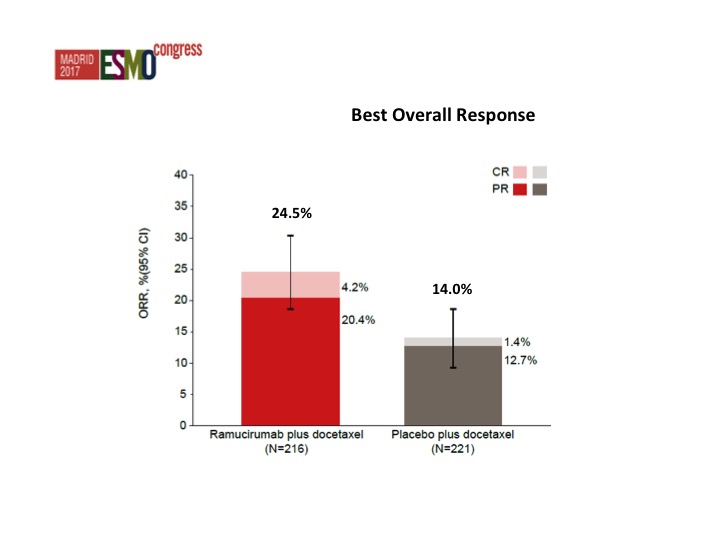
Nothing that stands out from the forest plots as far as any different subgroups are concerned but there was about a doubling of the objective response rate in favor of ram/docetaxel 24.5% versus 14%.
Treatment-emergent Adverse Events
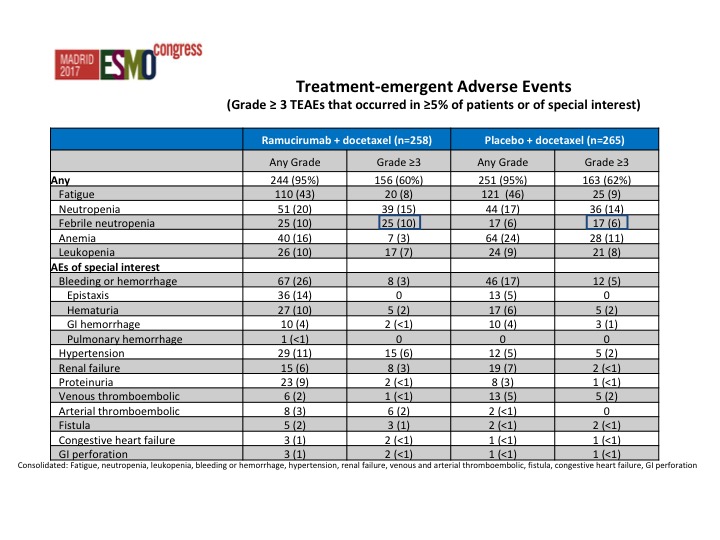
Strangely there was less toxicity in the combination arm, and this was actually somewhat surprising. In fact we saw less anemia in the combination arm than we did in the single-agent docetaxel arm.
Conclusions
We’ve concluded that there was an improvement in progression-free survival, improvement in objective response rate, and we just have to wait until we see the data from the survival.
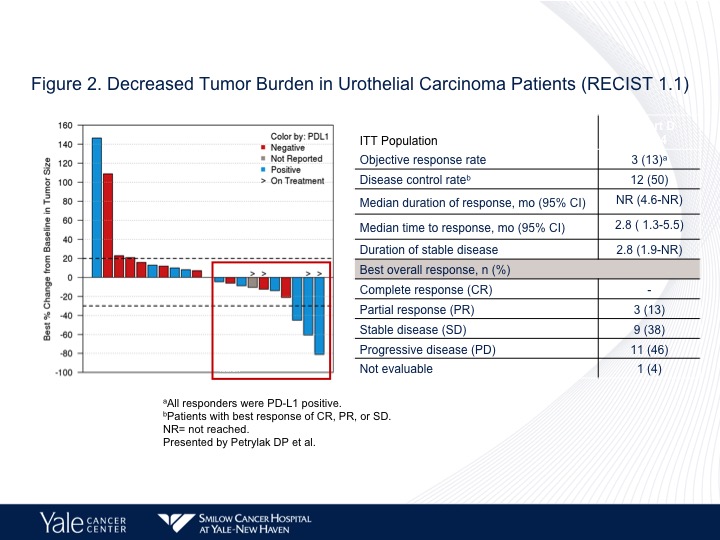
Figure 2
We’ve actually gone forward with ramucirumab in combination therapy. We combined it with pembro. This was a Phase 1 trial that we presented at ASCO GU this year. It’s currently being written up for publication, but nonetheless a little bit disappointing in the responses but very, very small trial just designed to look at toxicity to see whether we can combine the two, and the lung cancer data looks very, very good, but there are probably more patients on that.
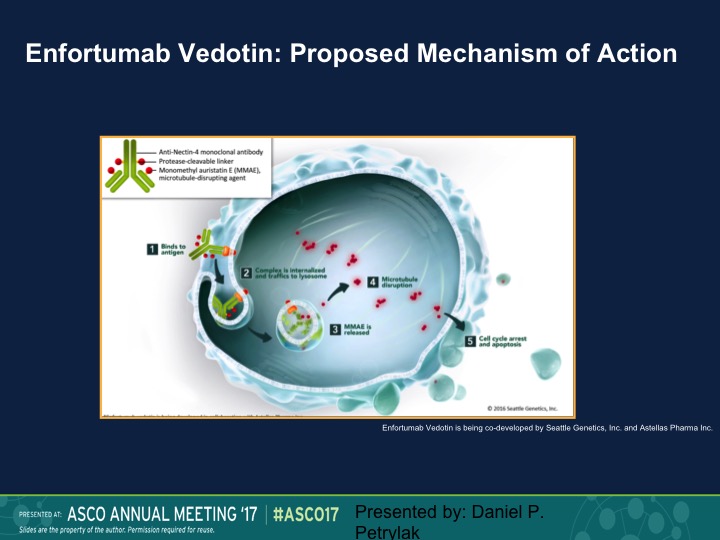
Enfortumab Vedotin: Proposed Mechanism of Action
Finally, the area that I think that I’m most excited about are these targeted ADS or antibody drug complexes. So enfortumab vedotin is a complex of a monoclonal antibody that’s linked to MMAE, which is methyl – – , which is an antitubulin agent. This enters the cancer cell via the nectin receptor. Nectin is expressed on about 90% of urothelial carcinoma specimens. It’s then cleaved, and the MAAE is released. It has an anti-tubulin effect. You get about a 1000-fold higher of a dose intracellular than you would if you gave this intravenously. In fact, you really can’t tolerate this drug intravenously well, and then it causes cell death and apoptosis.
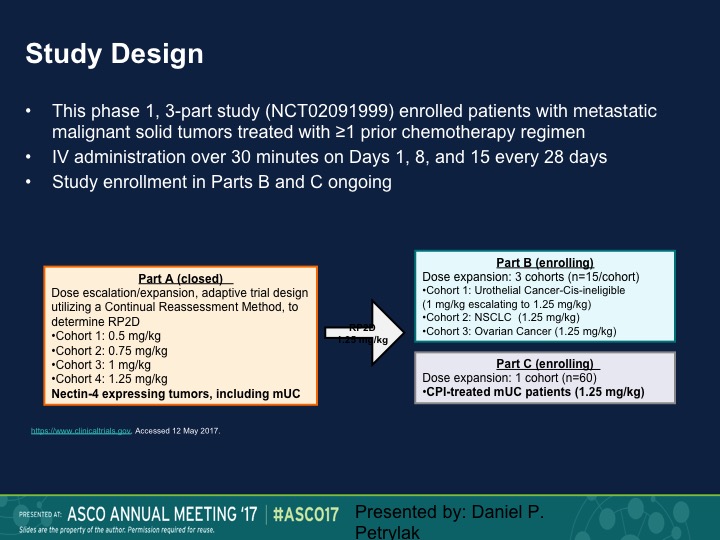
Study Design
So this was a Phase 1 trial. We presented this data at ASCO last year. We’re going to present an update at ASCO GU in a week, and we took patients standard dose escalation, start at 0.5 mg/kg, eventually round up at 1.25 mg/kg and then we did an expansion dose at that particular level. Nectin 4 as I mentioned before is expressed largely in urothelial carcinoma. In fact, we initially had nectin staining as a prerequisite for going on this trial. We dropped that because as I said before, 92% had nectin expression.
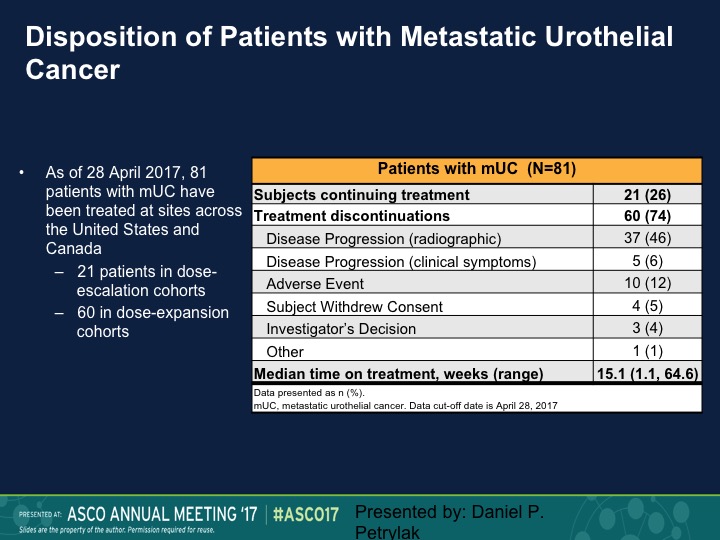
Disposition of Patients with Metastatic Urothelial Cancer
This is our disposition of the patients with metastatic urothelial carcinoma. As of that analysis we still had about a quarter of patients on treatment, and 37 had disease progression.
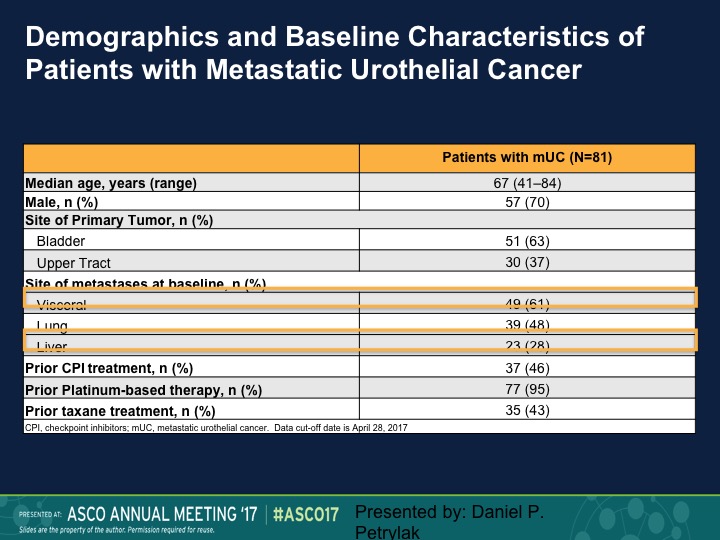
Demographics and Baseline Characteristics of Patients with Metastatic Urothelial Cancer
The median age of the patients was 67, predominantly bladder is their primary site, but we had actually a fairly large number of patients with visceral disease, and about a quarter of patients had liver metastases.
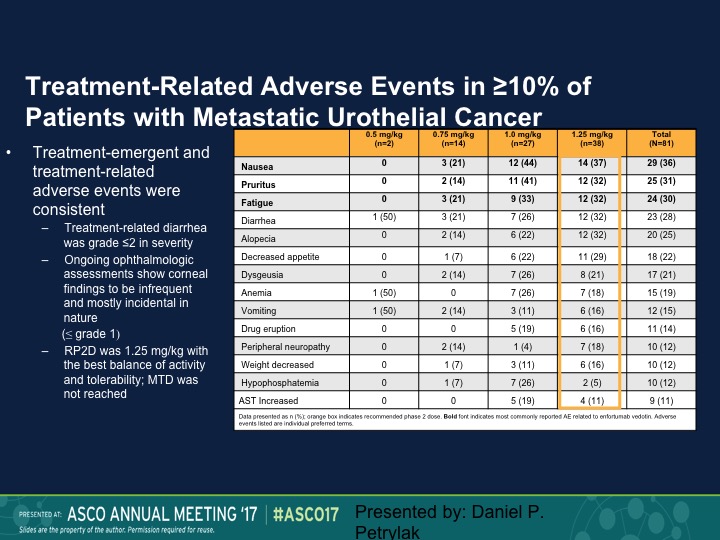
Treatment-Related Adverse Events
Fairly well tolerated. Neutropenia was seen. We also saw an increased risk of urinary tract infections. That necessarily was probably more due to the anatomic site of these tumors. We did see diarrhea. One of the expected toxicities, which was keratitis, we didn’t see, and patients who developed keratitis with these types of agents generally required steroid eye drops, but we didn’t see that at all with this particular complex.
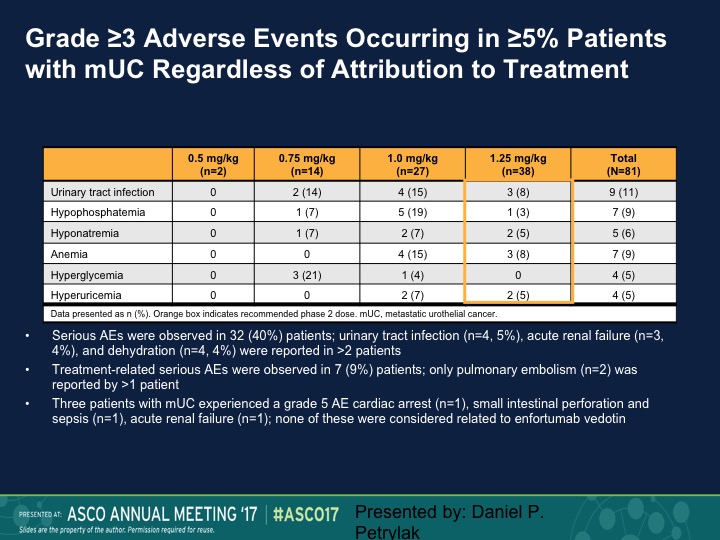
Grade ≥3 Adverse Events Occurring in ≥5% Patients with mUC Regardless of Attribution to Treatment
These are just some of the other side effects, urinary tract infection, I mentioned before, hyponatremia, hypophosphatemia.
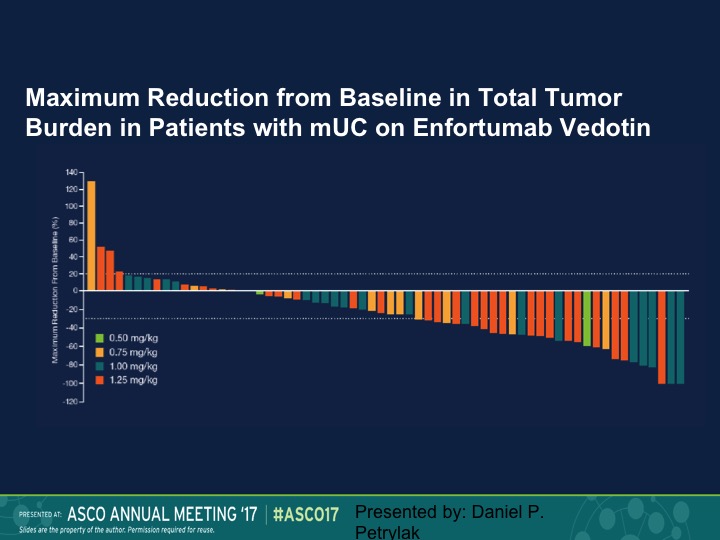
Maximum Reduction from Baseline to Total Tumor Burden in Patients with mUC on Enfortumab Vedotin
Here is our waterfall plot, and as we see here we saw responses at all dose levels. Again, this is going to be updated in two weeks.
Investigator-Assessed Response in Patients with mUC on Enfortumab Vedotin
And at different dose levels we had an objective response rate of 50% at 0.5 mg/kg, and 30% again complete responses were seen at the 1.4mg/kg dose. So again an active compound.
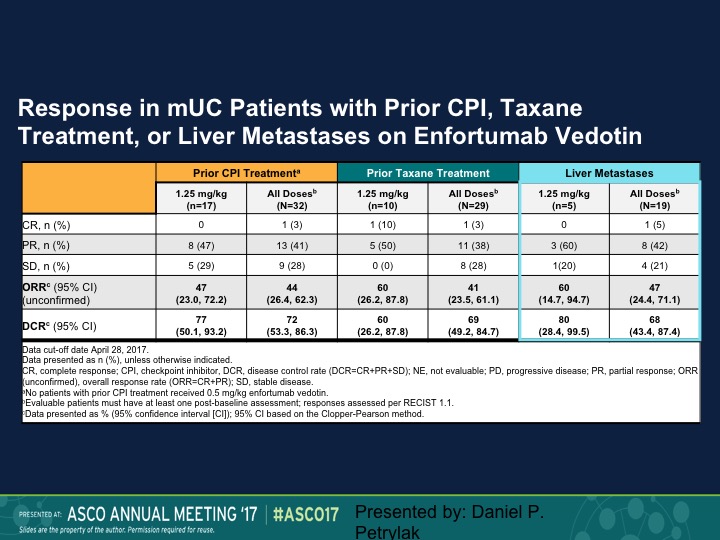
Response in mUC Patients with Prior CPI, Taxane Treatment, or Liver Metastases on Enfortumab Vedotin
And the question of course is does this make a difference in terms of whether these patients had prior therapy or not. So the 1.25 mg/kg is on the lower portion, 47% response rate in that group, 44% for all doses. When we look at prior taxane treatment it is about the same, and then liver metastases it’s 50%. So this is an area where we really don’t have a great deal of activity of checkpoint inhibition therapy. We’re going to update that data at ASCO GU and I can’t disclose that at this particular point, but we also saw about the same response rate in patients who had prior checkpoint therapy, about 50%.
Conclusions
It’s an exciting drug, and I think that it certainly could be a third line agent after checkpoint therapy or after RAM dosing, but I think that again we have a lot of different ways that we can improve this disease, which we didn’t have before. So we’re looking at multiple targets in urothelial carcinoma. These include EGF, AKT, and FGFR. Angiogenesis therapy combined with docetaxel improves progression-free survival in a phase 3 trial, and I think again one of the more exciting areas are these targeted monoclonal antibodies, and we’ll see more information. There are phase 3 trials ongoing. So I’ll stop there, and I think that if we don’t have any questions we’re up for a break.
ABOUT THE AUTHOR
Daniel P. Petrylak, MD, is currently Director of Genitourinary Oncology, Professor of Medicine and Urology, Co-Leader of Cancer Signaling Networks, and Co-Director of the Signal Transduction Program at Yale University Cancer Center in New Haven, Connecticut. He is a recognized international leader in the urology field. He earned his MD at Case Western Reserve University School of Medicine in Cleveland Ohio. He then went on to complete his Internal Medicine Residency at Albert Einstein College of Medicine/Jacobi Medical Center in the Bronx, and his fellowship at Memorial Sloan Kettering Cancer Center in New York.
Dr. Petrylak has served as principal investigator (PI) or co-PI on several SWOG clinical trials for genitourinary cancers. Most notably, he served as the PI for a randomized trial that led to the FDA approval of docetaxel in hormone refractory prostate cancer. He also helped to design and served as PI for the SPARC trial, an international registration trial evaluating satraplatin as a second-line therapy for hormone refractory prostate cancer.
Dr. Petrylak served on the program committees for the annual meetings of the American Urological Association from 2003-2011, and for the American Society of Clinical Oncology from 1995-1997 and 2001-2003. He also has served as a committee member for the Devices and Immunologicals section of the FDA. He has published extensively in the New England Journal of Medicine, Journal of Clinical Oncology, Journal of the National Cancer Institute, Cancer Research, and Clinical Cancer Research.