Localized Upper Tract Urothelial Carcinoma Managed with “Watchful Waiting”
How to cite: Schulam, Peter. “Localized Upper Tract Urothelial Carcinoma Managed with ‘Watchful Waiting’” February 7, 2018. Accessed [date today]. https://dev.grandroundsinurology.com/Localized-Upper-Tract-Urothelial-Carcinoma-Managed-with-Watchful-Waiting/
Summary:
Peter Schulam MD, PhD, compares and contrasts the Surveillance, Epidemiology, and End Results (SEER) database and the National Cancer Database (NCDB) in terms of reliability for assessing upper tract urothelial carcinoma (UTUC) outcomes and risk factors when determining whether to treat a patient with definitive therapy or the “Watchful Waiting” strategy.
Localized Upper Tract Urothelial Carcinoma Managed with “Watchful Waiting” – Transcript
Click on slide to expand
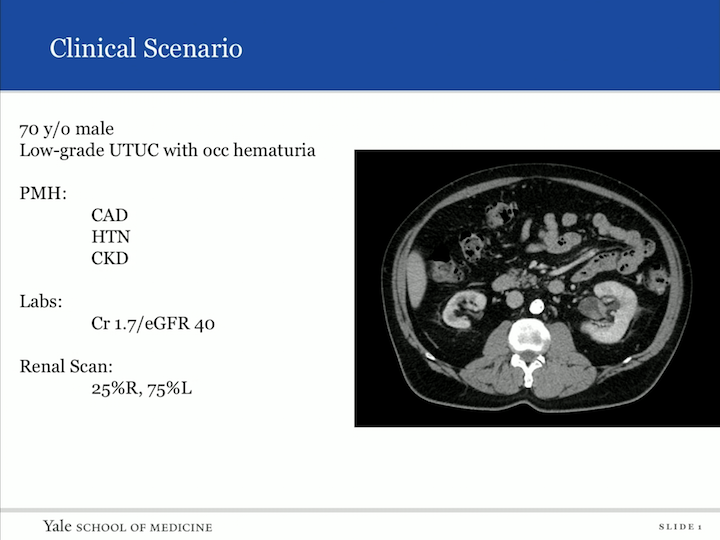
Clinical Scenario
So, I want to begin with a clinical scenario. So, patient comes into your clinic, 70-year-old male, low-grade upper tract urothelial carcinoma with occasion hematuria, biopsy proven. Past medical history: coronary artery disease, hypertension, chronic kidney disease. His creatinine is 1.7. His EGFR is 40. And you can look from the scan he has an atrophic right kidney, and unfortunately in the left, you can see in the renal pelvic, a TCC. You get a nuclear scan. It’s 25%, 75%, so now you’re sitting here realizing that if you do the gold standard of treatment and extirpative therapy, most likely this patient is going to be leading down the path of dialysis with a life expectancy less than five years. So what do you do, and how do you counsel him?
We have very little information available to us today regarding the natural history of upper tract urothelial carcinoma, and I think that’s the purpose of this presentation.
Background
Background: an estimated 5% to 10% of urothelial malignancies present in the upper urinary tract. In general, the primary form of therapy for non-metastatic upper tract is extirpative therapy, including ablation. In the last few decades, the incidence of upper tract has risen with an associated stage migration towards more localized tumors, as we’ve seen in renal cell carcinoma. And the median age of diagnosis of upper tract is actually 75 years old. So, we’re dealing with an elderly population.
Patients may not be candidates for radical surgery, as the case with our patient, due to poor baseline renal function and concern for progression of dialysis. Radical surgery is associated with risks, and patients may not be able to tolerate it. So, often, patients may not be eligible for segmental or endoscopic or extirpative therapy.
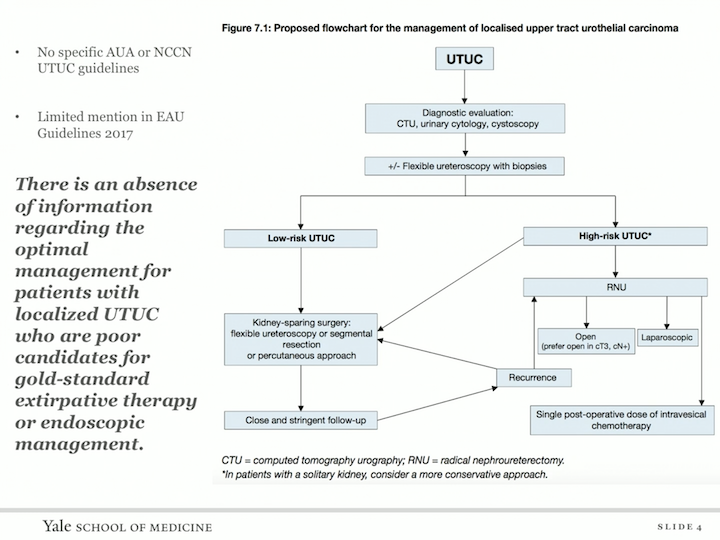
Proposed Flowchart for the Management of Localized Upper Tract Urothelial Carcinoma
Unfortunately, there’s an absence of information regarding the optimal management for patients with localized upper tract urothelial carcinoma who are poor candidates for gold standard extirpative therapy or endoscopic management, so this is the fact of both the AUW and the NCCN guidelines.
Background
So, the question is for those without symptoms, initial conservative approaches may be employed with consideration of system therapy upon symptomatic progression, so if you have someone who you are following conservatively, they begin to bleed, can you use embolization, can you use radiation. This strategy of watchful waiting with treatment upon distant progression has been adopted in other malignancies, including prostate cancer and in upper tract. This strategy could have the benefit of avoiding surgery while allowing treatment with chemotherapy or new immunotherapy agents, should disease progress. So, I know upper tract urothelial carcinoma is different than prostate cancers, different than renal cell, but the question is, we know now, we have second line immunotherapeutic agents that are effective against upper tract or urothelial carcinomas and may have a lower profile of harm to the patients, so in the future they may be available to us. So, we sought to determine the outcomes of patients with upper tract with non definitive therapy, a question that has not been addressed. We did this by interrogating two population data bases. The first was the surveillance epidemiology and results are the SEER database, and the other was the national cancer data base, the NCDB. So, the SEER database is a population based database that allows for cancer specific analysis. However, it does not have data regarding comorbidities and therapeutic modalities such as radiotherapy or chemotherapy, and the NCDB has surrogates for comorbidities and information regarding receipt of chemo and radiotherapy. However, it does not have data regarding cancer specific mortality. So, each of them has a plus and a minus.
Data from the SEER Program
So, we started with the SEER database and we assess the overall survival and cancer specific outcomes for patients with localized upper tract in a cohort of patients who did not receive any form of surgical therapy. We assess the risk factors for disease specific death, and we estimate the three year cancer specific mortality.
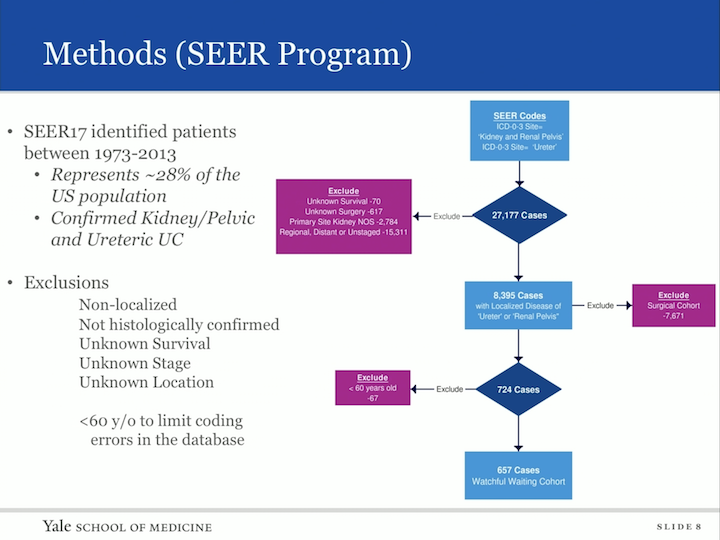
Methods (SEER Program)
Our methods- so we interrogated the SEER database that identified patients between 1973 and 2013. This represents 28% of the US population. All these patients were confirmed to have kidney, pelvic urothelial carcinoma. They are using the ICD code 03. We had many exclusions, non-localized histology was not confirmed, unknown survival state or location, and in fact, once we did this, there was a cohort of patients that were under the age of 60 that we felt as though we needed to remove from our cohort of urothelial tract, upper tract urothelial carcinoma who were not treated with definitive therapy, because we were concerned that there was coding errors, because usually these patients based on age alone would be given aggressive therapy.
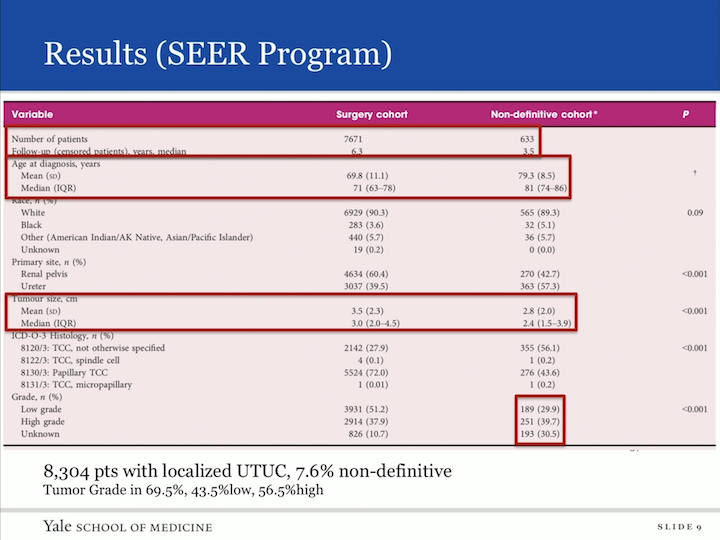
Results (SEER Program)
So here’s our data. In total, we found 8,304 patients who had localized upper tract urothelial carcinoma in the SEER database. We had 633 of these patients who did not receive definitive therapy, so there was a 7.6% group within the SEER database that received non-extirpative therapy or were followed. The age of diagnosis, as you can imagine, in non-definitive are not in the treatment lacking group was their older and their tumors were, in fact, smaller. Of that group that was non-definitive, 69.5% actually had a grade associated, and when you went ahead and looked at that data, 43% had low grade and 56% had high grade upper tract urothelial carcinoma, so then we did our analysis on this cohort.
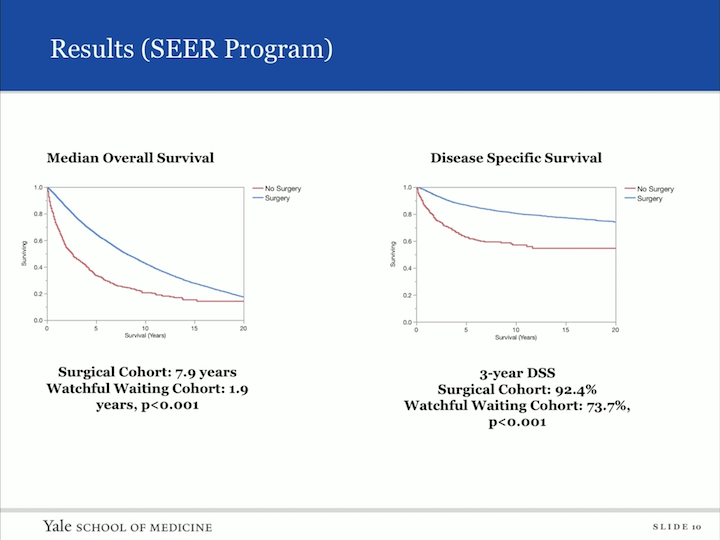
Results (SEER Program)
So, we looked at median overall survival, comparing those who had surgery to those who did not have surgery, and as you can imagine, the surgical cohort had a longer overall survival of 7.9 years versus watchful waiting of 1.9 years. We then looked at the disease specific survival, and the three year disease specific survival, again, showed the surgical cohort at 92.4%, but interesting to us, the watchful waiting cohort was actually 73.7% three year disease specific survival.
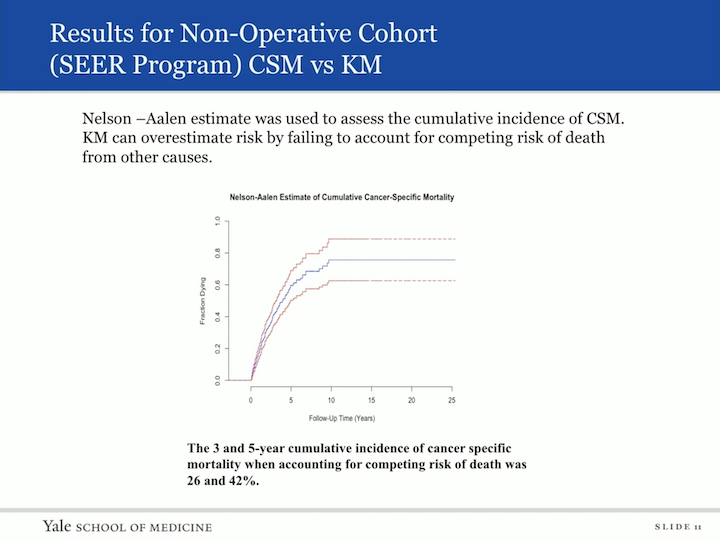
Results for Non-Operative Cohort (SEER Program) CSM vs KM
We then wanted to look at actually the cancer specific mortality, and in order to do this, we used a different tool. The Nelson L&S was used to assess the cumulative incidence of cancer specific mortality. The Kaplan-Meier curves can overestimate risks by failing to account for competing risk of deaths from other causes, so when we applied this tool to our data set, the three and five year cumulative incidence of cancer specific mortality, when accounting for competing risks of death, was 26% and 42%, so the risk of death to someone with an untreated urothelial carcinoma was only 26% at three years.
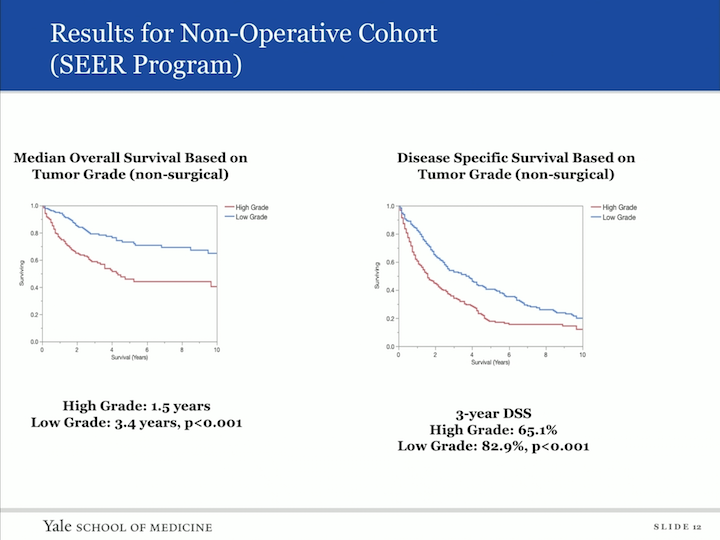
Results for Non-Operative Cohort (SEER Program)
We then did a subset analysis. So, we looked at those patients who did not have definite therapy and asked the question, “What was the effect of grade on their overall survival?” And that’s in this panel, and again, you can image those patients who had high grade disease. They had a shorter overall survival, with 1.5 years versus low grade at 3.4 years, and then similarly, in the disease specific survival high grade of 65.1%, and low grade was 82.9% survival at three years. We also did a multivariate model hazard ratio analysis to look at predictors of worse survival, and age and high tumor grade fell out as indicators of worse prognosis.
Localized upper tract urothelial carcinoma managed conservatively
So, then we did a similar analysis on the NCDB database.
Data from the NCDB
We were looking at overall survival with the following stratifications. We looked at grade, Charlson-Dyeo Comorbidity Score, single or multiagent chemotherapy, or radiation. Remember in the NCDB data base we get, we actually have information regarding those who received chemotherapy and radiation versus SEER database, and cancer specific survival was not available in the NCDB.
Methods (NCDB)
So, the NCDB data base identified patients between 2004 and 2014 represents 70% of all cancer cases from over 1500 cancer programs in the United States. Again, we used a similar code, so we’re looking at renal pelvic, ureter, upper tract urothelial carcinoma. Our exclusions, again, unknown surgical status not histologically confirmed. Unknown survival, unknown state, and unknown location.
Results (NCDB)
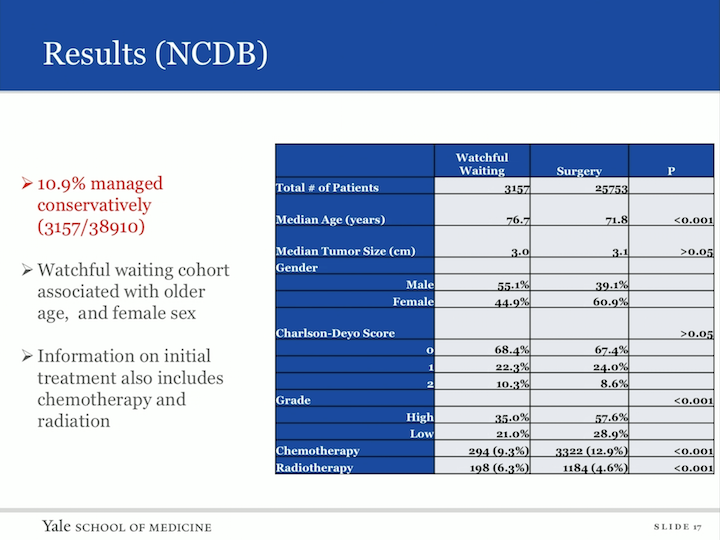
So, our cohort fell out. We actually had 38,910 patients with upper tract urothelial carcinoma in this database. 3157 were managed conservatively, so 10.9% of the cohort. The watchful waiting cohort was associated with older age and female sex, and for the first time, we were actually able to identify information on initial treatment with chemo or radiation in a group that did not receive extirpative therapy, and in that cohorts are 9.3% in the watchful waiting group who received chemotherapy, 6.3% received radiation therapy.
Results (NCDB)
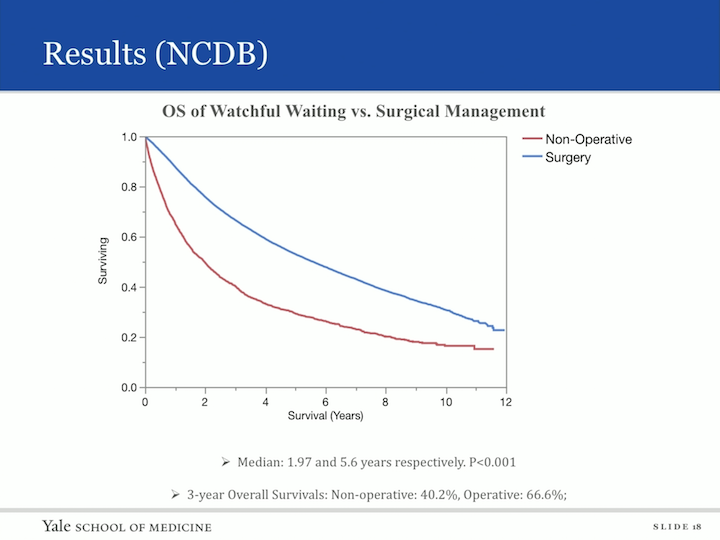
Again, we looked at overall survival and similarly as to the SEER database when comparing nonoperative to surgical. The median survival for those who did not have surgery was two years, and those who did receive surgery was 5.6 years. The three year overall survival nonoperative was 40.2% and operative was 66.6%.
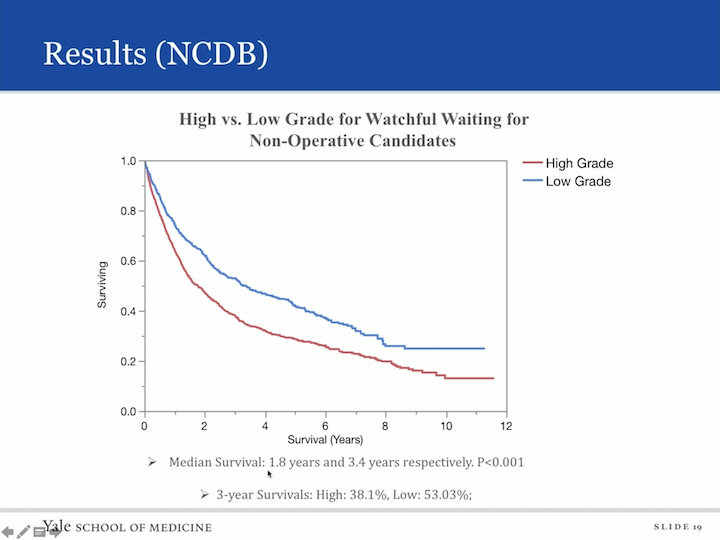
Results (NCDB)
And if we did subset analysis, again, looking at high grade and low grade, again not surprisingly, those with low grade disease had a median survival of 3.4 years versus 1.8 years in those men and women with low grade disease, I’m sorry, with high grade disease, and the three year survival high grade was 38% and low grade 53%. We also asked the question, “What was the effect of the Charlson score on patients?” And not surprisingly, those who had an increasing Charlson score also, so therefore, increasing morbidity had decreasing survivability.
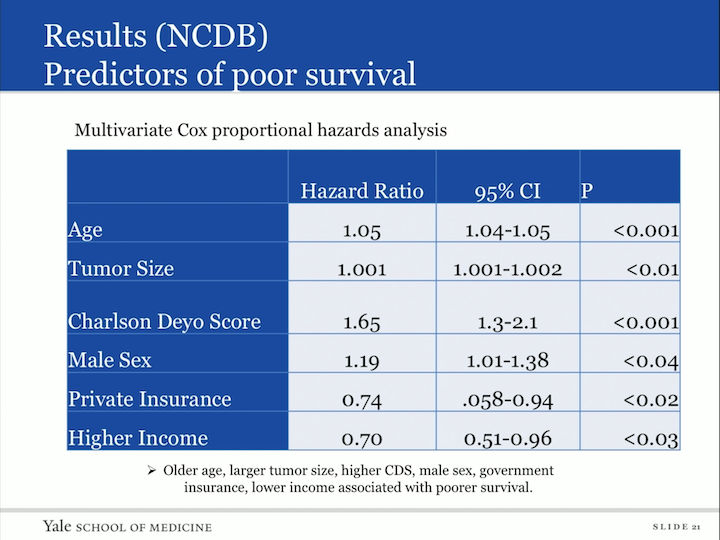
Results (NCDB) Predictors of Poor Survival
We also did a multivariate Cox proportional hazards analysis, looked at predictors or poor survival and what fell out as indicators of poor survival was older age, larger tumor, a higher Charlson score, male sex, those who had government insurance versus private, and those patients with lower income.
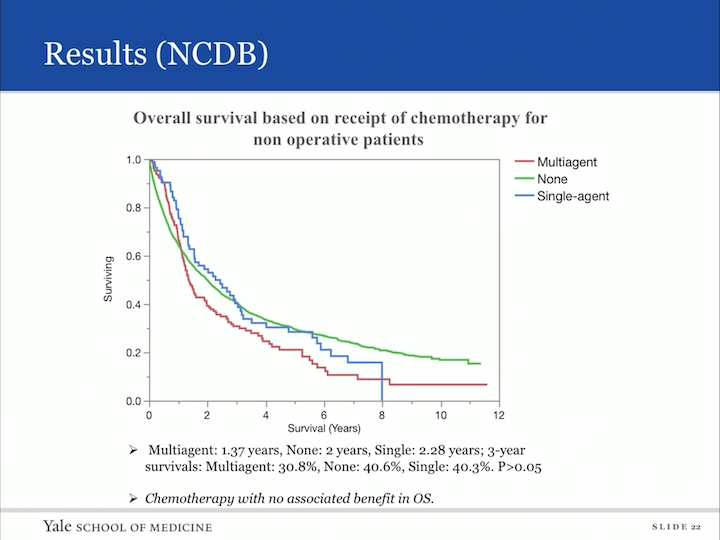
Results (NCDB)
Now, I mentioned we were able to get some information regarding chemotherapy and radiotherapy and this is the non-definitive group. So, you can imagine, you don’t want to operate, but can you do something else, so this is a cohort that had received either multiagent, no chemotherapy or single agent, but all of these did not receive surgery. And what we found was that chemotherapy had no associated benefit to overall survival in the group.
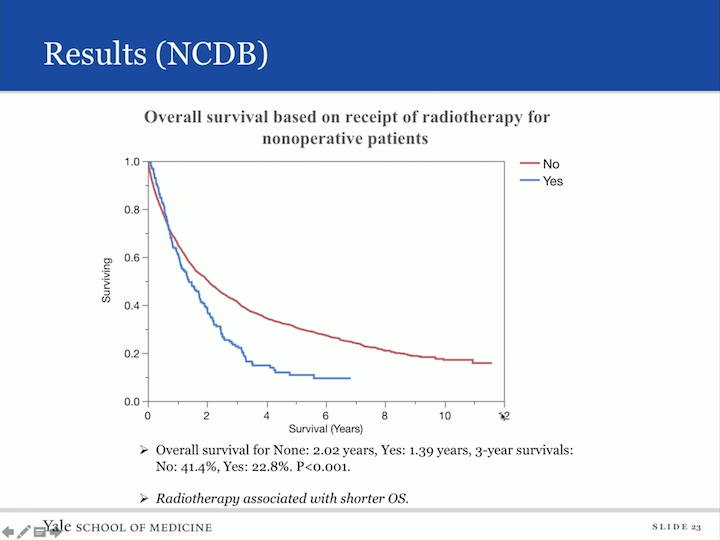
Results (NCDB)
When we did a similar analysis to radiotherapy, interestingly enough, radiotherapy was associated with a shorter overall survival. How could that be? I’m sure what the case is that these are patients with aggressive disease, not surgical candidates. You’re throwing the kitchen sink at them, radiation may be it, and basically, they have poor survival.
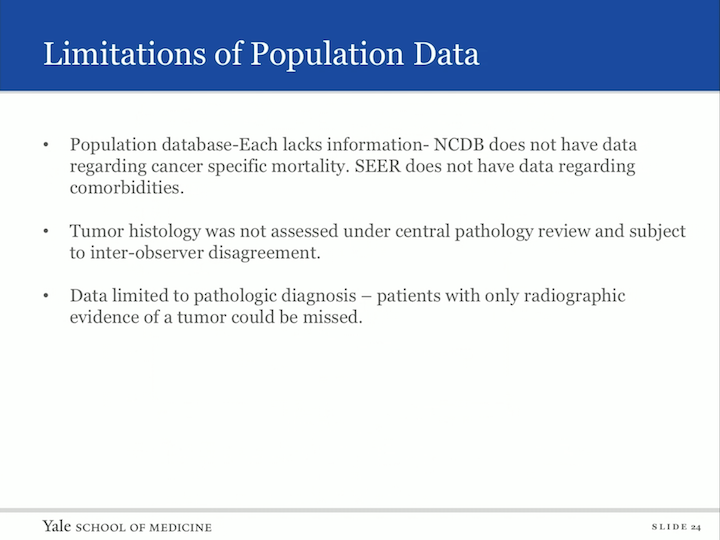
Limitations of Population Data
The limitations of the study is that this is a population data base. Each lacks information. NCDB does not have data regarding cancer specific mortality. SEER does not have data regarding comorbidities. Tumor histology was not assessed under central pathology review, so we’re subject to inter-observer disagreement and the data is limited to pathologic diagnosis, so those patients who only had a radiologic diagnosis were not included in this study.
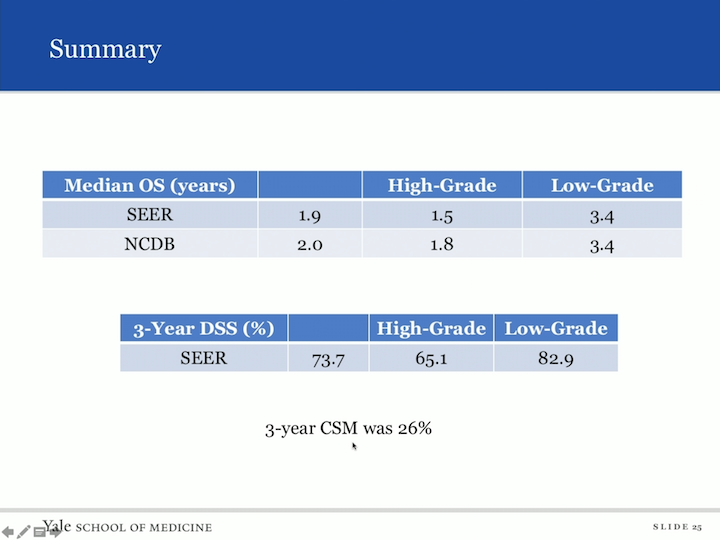
Summary
But here’s the summary. So, you look at the SEER database and look at median overall survival. It was 1.9 years. If you had high grade, it was 1.5 and it was as long as 3.4 in low grade. In the NCDB database, we found a similar number for overall survival of two years. High grade was 1.8 years and again low grade was 3.4 years. Disease specific survival, we’re not as able to assess an NCDB, but the SEER database gave us a three year disease specific survival of 73.7. High grade was associated with 65.1%, but low grade was as high as 82.9% and the three year cancer specific mortality was 26%.
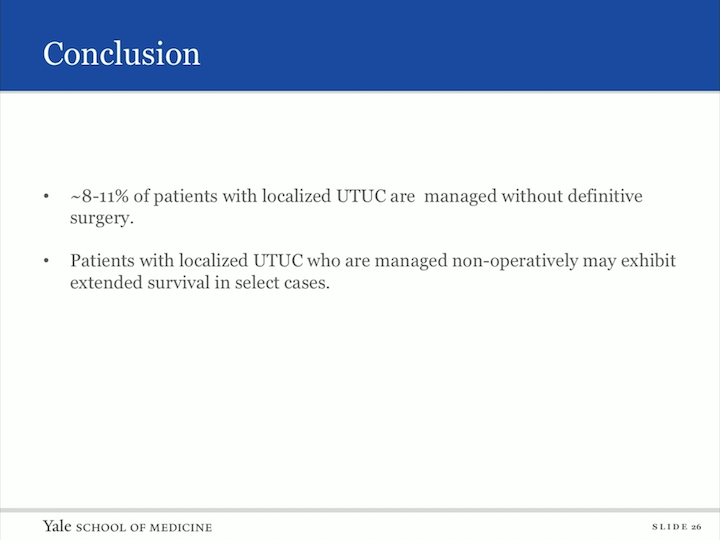
Conclusion
So, in conclusion, 8%-11% of our patients in both databases had localized upper tract urothelial carcinoma that was managed without definitive therapy. The patients with localized upper tract urothelial carcinoma who were managed nonoperatively may exhibit extended survival in select cases. The age of diagnosis in the tumor grade impact cancer specific mortality of the patients with localized upper tract managed nonoperatively from the NCDB data base 9.3% and 6.3% received chemotherapy and radiotherapy respectively. XRT and chemotherapy may not have overall survival benefit in this population, and further research is needed to define which borderline t6x candidates can be safely treated conservatively.
Clinical Scenario
So, let’s go back to that clinical scenario of our 70 year old gentleman with a low grade upper tract urothelial carcinoma with significant past medical history who has chronic kidney disease and an atrial right kidney. How can you counsel this patient? Is there more information that you can tell that patient? I think, based on this study, we can now counsel the patient regarding conservative management in that there is a 20% to 25% risk of cancer related death at three years.
ABOUT THE AUTHOR
Dr. Peter G. Schulam is Professor and Chair of the Department of Urology at Yale School of Medicine. He also serves as Chief of Urology at Yale New Haven Hospital and Chief Innovation and Transformation Officer of the Yale New Haven Health System. He is Faculty Director of the Tsai Center for Innovative Thinking at Yale, and Co-Founder of the Center for Biomedical Innovation and Technology.
Dr. Schulam graduated from the Medical Scientist Training Program at Baylor College of Medicine with an MD and a PhD in Immunology and completed his surgical and urologic training at Johns Hopkins Hospital in Baltimore, Maryland. Following a brief period at Baylor in Houston, he was recruited as the Chief of Minimally Invasive Surgery in the Department of Urology at UCLA. At UCLA, he developed laparoscopic and robotic surgery programs for prostate and kidney cancer. Dr. Schulam was also the Surgical Director of the Living Kidney Donor Program and performed over 1,200 laparoscopic living donor kidney procedures. At UCLA, he became the vice chair of the department and was actively involved in improving the patient experience throughout the hospital.
In January of 2012, he became the Chair of the Department of Urology at Yale. Dr. Schulam’s focus is to build a program that can offer every patient with any urologic condition state of the art diagnosis and treatment in an environment that is patient centered and is committed to listening and engaging the patient in their care.