Daniel P. Petrylak, MD, presented “Checkpoint Inhibitors for NMIBC” during the 27th Annual Perspectives in Urology: Point Counterpoint on November 9, 2018 in Scottsdale, Arizona.
How to cite: Petrylak, Daniel P. “Checkpoint Inhibitors for NMIBC” November 9, 2018. Accessed Nov 2024. https://dev.grandroundsinurology.com/checkpoint-inhibitors-for-nmibc/
Checkpoint Inhibitors for NMIBC – Summary:
Daniel P. Petrylak, MD, provides an overview of immunotherapy treatment options for non-muscle invasive bladder cancer (NMIBC) including BCG, intravesical therapies, and checkpoint inhibitors. He also discusses clinical trials investigating alternative immunotherapy options for NMIBC other than BCG.
Overview of NMIBC Treatment
Non-muscle invasive bladder cancer (NMIBC) encompasses about 75-85% of bladder cases. Unfortunately, it is an expensive disease to treat due to the long term management needed. However, a main goal for physicians treating NMIBC is to avoid cystectomy. Avoiding cystectomy improves patient quality of life and averts large healthcare costs. Unfortunately, radical cystectomy is the standard of care treatment for BCG refractory, high-grade NMIBC.
Nevertheless, there are other agents, including new immunology-based therapies, in development and undergoing clinical trials for NMIBC. Other options in this setting include Intravesical therapies, such as chemotherapies, vaccines, and viruses. Additionally, the Southwest Urology Group (SWOG) is investigating a different form of BCG: Tokyo-172 strain BCG. The shortage of BCG largely influenced this investigation. SWOG is also evaluating T-cell priming in BCG-naive, high-grade NMIBC (SWOG 1602).
Checkpoint Inhibitors for NMIBC
Checkpoint inhibitors have activity in a variety of different cancers, such as lung and bladder cancer. Generally, cancer types with high mutational rates respond well to checkpoint inhibition. Pembrolizumab and atezolizumab are checkpoint inhibitors with FDA-approval for first-line therapy in cisplatin-ineligible metastatic urothelial carcinoma (mUC).
For NMIBC, patients who are BCG-resistant may have upregulation of PD-1. In the KEYNOTE-057 study, NMIBC patients who failed BCG received pembrolizumab every 3 weeks. The results of the study showed that almost 40% of patients with pembrolizumab had a complete tumor response. Also, no patients progressed from T2 disease in the group treated with pembrolizumab, and only 8.7% of patients had stage progression.
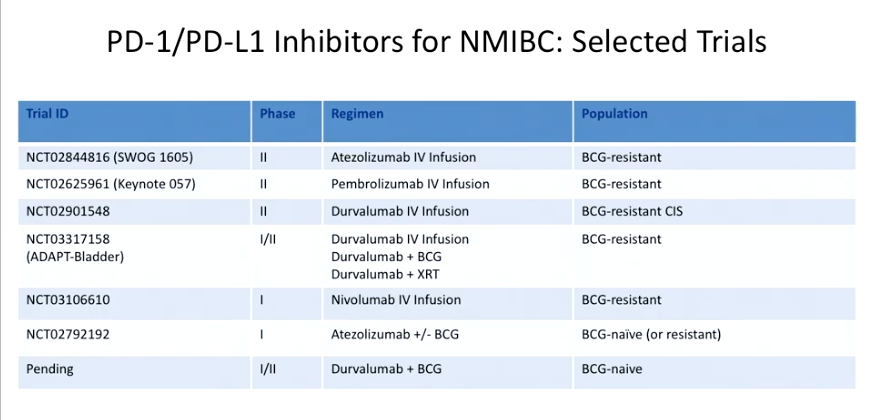
Other trials, as seen in the table to the left, are investigating checkpoint inhibitors in BCG-resistant, as well as BCG-naive, patients. The BCG-naive studies are focusing on combining checkpoint inhibitors with BCG.
Overall, high mutational load makes NMIBC an ideal target for immunotherapy. BCG immunotherapy is the standard of care for NMIBC. There is limited utility of chemotherapy for NMIBC, but there are clinical trials assessing the role of checkpoint inhibitors in NMIBC.
About Perspectives in Urology: Point Counterpoint
Perspectives in Urology: Point Counterpoint (PCP) is an annual CME-accredited conference devoted to discussing and debating the latest topics in men’s health, general urology, and genitourinary cancers. The conference’s format includes more than didactic lectures. It also includes debates, point-counterpoint discussion panels, and unique case-based presentations. Dr. Petrylak presented this lecture during the 27th PCP in 2018. Please visit this page in order to register for future PCP meetings.
ABOUT THE AUTHOR
Daniel P. Petrylak, MD, is currently Director of Genitourinary Oncology, Professor of Medicine and Urology, Co-Leader of Cancer Signaling Networks, and Co-Director of the Signal Transduction Program at Yale University Cancer Center in New Haven, Connecticut. He is a recognized international leader in the urology field. He earned his MD at Case Western Reserve University School of Medicine in Cleveland Ohio. He then went on to complete his Internal Medicine Residency at Albert Einstein College of Medicine/Jacobi Medical Center in the Bronx, and his fellowship at Memorial Sloan Kettering Cancer Center in New York.
Dr. Petrylak has served as principal investigator (PI) or co-PI on several SWOG clinical trials for genitourinary cancers. Most notably, he served as the PI for a randomized trial that led to the FDA approval of docetaxel in hormone refractory prostate cancer. He also helped to design and served as PI for the SPARC trial, an international registration trial evaluating satraplatin as a second-line therapy for hormone refractory prostate cancer.
Dr. Petrylak served on the program committees for the annual meetings of the American Urological Association from 2003-2011, and for the American Society of Clinical Oncology from 1995-1997 and 2001-2003. He also has served as a committee member for the Devices and Immunologicals section of the FDA. He has published extensively in the New England Journal of Medicine, Journal of Clinical Oncology, Journal of the National Cancer Institute, Cancer Research, and Clinical Cancer Research.