Daniel P. Petrylak, MD, presented “Immunotherapy for PCa: Where Do We Go From Here?” during the 29th Annual International Prostate Cancer Update on January 26, 2019 in Beaver Creek, Colorado.
How to cite: Petrylak, Daniel P. “Immunotherapy for PCa: Where Do We Go From Here?” January 26, 2019. Accessed Nov 2024. https://dev.grandroundsinurology.com/immunotherapy-for-pca-where-do-we-go-from-here/
Immunotherapy for PCa: Where Do We Go From Here?- Summary:
Daniel P. Petrylak, MD, discusses patient selection for currently-approved immunotherapies for prostate cancer, especially in patients with microsatellite instability. He also emphasizes the importance of utilizing immunotherapy early in the disease course.
Utilizing Immunotherapy Early in the Disease Course
Prostate cancer responds differently to immunotherapy than other diseases. For instance, about a quarter of bladder cancer patients will show dramatic objective responses to immunotherapy. While prostate cancer patients generally show a survival benefit with immunotherapy treatment, frequently patients do not respond. This is because the activation of the immune system may not initially activate after treatment. After that initial time period, immunotherapy may cause continued cumulative slowing pressure of the PSA curve. Therefore, there is rationale for using immunotherapy early in the disease course.
Before the very recently-available immunotherapies for prostate cancer, sipuleucel T was the most well-known option. Sipuleucel T is an FDA-approved agent for asymptomatic prostate cancer patients with bone disease and do not have visceral metastasis.
The IMPACT trial demonstrated a survival benefit in favor of sipuleucel T in mCRPC patients compared to placebo. An evaluation of this data found that patients with a baseline PSA level of 22.1ng/ML or less had a better survival benefit compared to the patient population with higher PSA. This supports the notion of administering immunotherapy earlier in the disease course. Additionally, a randomized, phase II trial investigating sipuleucel T plus concurrent or sequential enzalutamide found that survival and clinical outcomes were optimal when mCRPC patients received treatment earlier in the course of disease.
Microsatellite Instability and Immunotherapy
It is important to consider testing for mismatch repair, or microsatellite instability, when determining the best course of treatment for individual prostate cancer patients. The one drug with FDA approval across all tumor types is pembrolizumab, a PD-1 inhibitor. It shows an improvement in survival in patients with mismatch repair (MMR) regardless their tumor type. Therefore, clinicians should look for MMR in all patients using next-generation sequencing to take advantage of a possible dramatic response to pembrolizumab.
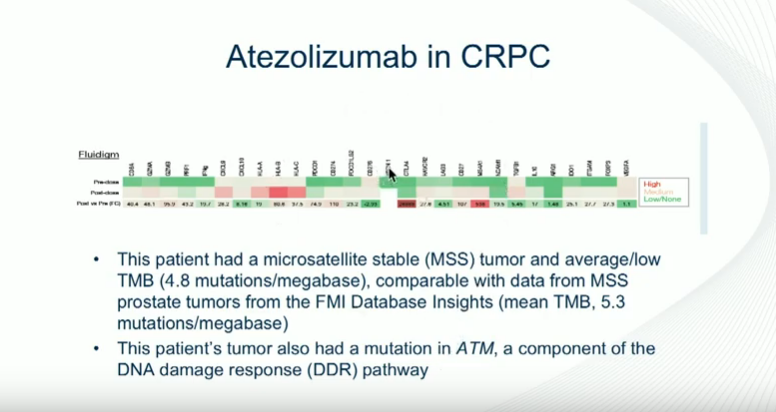
On the other hand, in a study investigating atezolizumab, a PD-L1 inhibitor, one patient had an extremely dramatic response, developing infiltration of his tumor with CD8 cells. Interestingly, his PD-L1 expression was negative when he started treatment. This patient did not have microsatellite instability but had a DNA repair mutation. Unfortunately, most of the patients in this study did not respond to atezolizumab.
This corresponds with this theme of immunotherapy for prostate cancer that patients who tend to respond well have DNA repair mutations. Therefore, testing for microsatellite instability and DNA repair mutations can help guide clinician’s choice in whether or not to use immunotherapy for prostate cancer patients.
About the International Prostate Cancer Update
The International Prostate Cancer Update (IPCU) is an annual, multi-day CME conference focused on prostate cancer treatment updates. The conference’s faculty consists of international experts and caters to urologists, medical oncologists, radiation oncologists, and other healthcare professionals. Topics encompass prostate cancer management, from diagnosis to treating advanced and metastatic disease. Dr. Petrylak presented this lecture during the 29th IPCU in 2019. Please visit this page in order to learn more about future IPCU meetings.
ABOUT THE AUTHOR
Daniel P. Petrylak, MD, is currently Director of Genitourinary Oncology, Professor of Medicine and Urology, Co-Leader of Cancer Signaling Networks, and Co-Director of the Signal Transduction Program at Yale University Cancer Center in New Haven, Connecticut. He is a recognized international leader in the urology field. He earned his MD at Case Western Reserve University School of Medicine in Cleveland Ohio. He then went on to complete his Internal Medicine Residency at Albert Einstein College of Medicine/Jacobi Medical Center in the Bronx, and his fellowship at Memorial Sloan Kettering Cancer Center in New York.
Dr. Petrylak has served as principal investigator (PI) or co-PI on several SWOG clinical trials for genitourinary cancers. Most notably, he served as the PI for a randomized trial that led to the FDA approval of docetaxel in hormone refractory prostate cancer. He also helped to design and served as PI for the SPARC trial, an international registration trial evaluating satraplatin as a second-line therapy for hormone refractory prostate cancer.
Dr. Petrylak served on the program committees for the annual meetings of the American Urological Association from 2003-2011, and for the American Society of Clinical Oncology from 1995-1997 and 2001-2003. He also has served as a committee member for the Devices and Immunologicals section of the FDA. He has published extensively in the New England Journal of Medicine, Journal of Clinical Oncology, Journal of the National Cancer Institute, Cancer Research, and Clinical Cancer Research.