Alan H. Bryce MD, presented “Adjuvant Therapy in High Risk Renal Cell Carcinoma” during the 27th Annual Perspectives in Urology: Point Counterpoint on November 9, 2018 in Scottsdale, Arizona.
How to cite: Bryce, Alan H. “Adjuvant Therapy in High Risk Renal Cell Carcinoma” November 9, 2018. Accessed Dec 2024. https://dev.grandroundsinurology.com/adjuvant-therapy-in-high-risk-renal-cell-carcinoma/
Adjuvant Therapy in High Risk Renal Cell Carcinoma Summary:
Alan H. Bryce, MD, surveys data from three phase III trials on adjuvant therapies for high-risk renal cell carcinoma. He discusses the controversies following the conclusions of the completed trials and emphasizes the need for research in further adjuvant therapies beyond those that target the VEGF pathway.
The Need for Adjuvant Therapies in Renal Cell Carcinoma
About 15%-20% of renal cell carcinoma (RCC) patients present with high-risk locoregional disease. This carries a poor 5-year overall survival (OS) rate for Stage III disease, at around 50%-60%. Subsequently, there is a motivation to improve treatments for these patients. In the metastatic RCC setting, the FDA has approved a variety of agents since 1992. Also, anti-vascular endothelial growth factor (VEGF) tyrosine-kinase inhibitors (TKIs) have proven to provide OS benefit in metastatic disease. Because of this, there is interest in moving these agents forward to the adjuvant setting.
Completed Trials for Adjuvant Therapies
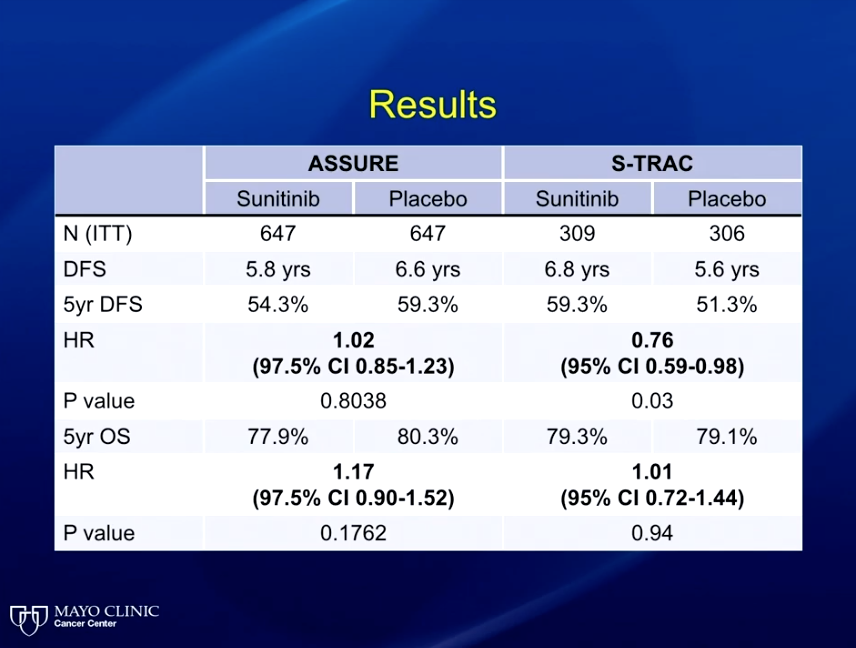
Of the approved agents for metastatic RCC, phase III trials have reported on sunitinib, sorafenib, and pazopanib.
The ASSURE trial randomized patients to sunitinib, sorafenib, or placebo and showed no difference in progression-free survival (PFS) between the three arms. Contrastly, the S-TRAC trial investigated sunitinib against placebo and showed a clinically-significant PFS benefit to sunitinib. A meta-analysis of this data demonstrated that sunitinib resulted in significant toxicities, and therefore inferior quality of life outcomes for patients.
This meta-analysis also noted four treatment-related deaths. The discrepancies in trial designs between ASSURE And S-TRAC substantiated reasoning that the differing results could be due to the idea that sunitinib could be effective in high-risk patients, but inappropriate for low-risk patients. However, an unplanned subset analysis of ASSURE evaluated sunitinib in high-risk patients and still found no significant benefit.
The PROTECT Trial looked at pazopanib in the adjuvant setting. During this study, the side-effects of treatment led to massive participant attrition. In response, the investigators readjusted the trial from an 800mg dose to a 600mg dose. Ultimately, the trial concluded that the toxicity of pazopanib at full dose was difficult for patients to tolerate in the adjuvant setting, and the 600mg dose showed no disease-free survival (DFS) or OS benefit.
Future Directions
While sunitinib is the only agent with FDA approval for high-risk adjuvant RCC, adoption amongst oncologists is low. When deciding whether or not to recommend sunitinib in this setting, a 12-member Oncologic Drugs Advisory Committee (ODAC) panel split 6-6 on the issue. There are ongoing studies into additional adjuvant therapies, namely everolimus, an MTOR inhibitor, as well as a nivolumab plus ipilimumab combination, pembrolizumab, and atezolizumab. Future research about better adjuvant therapies should continue, especially those which do not target the VEGF pathway, focusing on a goal to cure.
About Perspectives in Urology: Point Counterpoint
Perspectives in Urology: Point Counterpoint (PCP) is an annual CME-accredited conference devoted to discussing and debating the latest topics in men’s health, general urology, and genitourinary cancers. The conference’s format includes more than didactic lectures. It also includes debates, point-counterpoint discussion panels, and unique case-based presentations. Dr. Bryce presented this lecture during the 27th PCP in 2018. Please visit this page in order to register for future PCP meetings.
ABOUT THE AUTHOR
Dr. Bryce is the Medical Director of the Genomic Oncology Clinic at Mayo Clinic Arizona in Scottsdale, where he utilizes whole genome sequencing of tumors to identify key driver mutations. This approach allows for precise targeting of a patient’s tumor, leading to a greater chance of remission.
Dr. Bryce received a BS in Biochemistry from the University of California, Los Angeles. He then went on to receive his MD from Finch University of Health Sciences/Chicago Medical School. He completed a residency at the Mayo Graduate School of Medicine, and then received a Fellowship in Hematology/Oncology, also at the Mayo Graduate School of Medicine. He eventually served as Chief Fellow of Hematology/Medical Oncology there.
Dr. Bryce studies cancer genetics and novel therapeutics with a focus on personalized medicine. His clinical practice centers on genitourinary malignancies (prostate, kidney, bladder, and testicular cancers) and melanoma. In addition, Dr. Bryce participates in community outreach to underserved populations and has an interest in health disparities research. He also conducts Phase I clinical trials of new cancer drugs.