Dr. Sam S. Chang, MD, MBA, presented “Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA-ASCO-ASTRO-SUO Guidelines” at the 2nd Annual International Bladder Cancer Update on January 27, 2018 in Beaver Creek, Colorado
How to cite: Chang, Sam S. “Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA-ASCO-ASTRO-SUO Guidelines” January 27, 2018. Accessed Dec 2024 . https://dev.grandroundsinurology.com/aua-asco-astro-suo-guidelines/
Summary:
Sam Chang, MD, MBA, who has served as chair of the American Urological Association (AUA) guidelines panel for non-muscle invasive bladder cancer (NMIBC) and muscle invasive bladder cancer (MIBC), provides an overview of updated non-metastatic MIBC treatment guidelines, including a comprehensive treatment algorithm. He also discusses directions of future research for the disease.
Reveal the Answer to Audience Response Question #1
The bladder-sparing regimen that is most likely to be curative for invasive bladder cancer is:
- A. Partial cystectomy and BPLND
- B. Maximal TURBT and chemotherapy
- C. XRT
- D. Maximal TURBT, chemotherapy, and XRT
(Twitter Question) Reveal the Answer to Audience Response Question #2
Optimal neoadjuvant chemotherapy would include:
- A. mitomycin C
- B. atezolizumab
- C. Carboplatin
- D. Cisplatin
Reveal the Answer to Audience Response Question #3
For decreased length of stay after radical cystectomy, the medication of choice is:
- A. erythromycin
- B. phenergan
- C. reglan
- D. alvimopan
Treatment of Non-Metastatic Muscle-Invasive Bladder Cancer: AUA-ASCO-ASTRO-SUO Guidelines – Transcript
Click on slide to expand
Treatment of Non-Metastatic Muscle Invasive Bladder Cancer: AUA/ASCO/ASTRO/SUO Guidelines
These are the guidelines that came out actually just this fall. Actually several members here were involved looking at treatment – – for non-metastatic muscle-invasive disease.
Purpose
So the purpose here was again similar to the other guidelines that had been outlined to look at a framework then for not only urologists but for medical oncologists and radiation oncologists as well, to include for the treatment outline for those patients that then have non-metastatic disease.
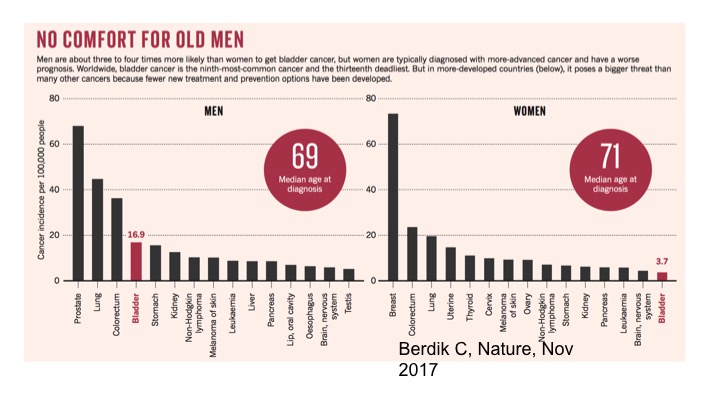
No Comfort For Old Men
We understand both for men and women, but specifically looking at the curves for men in terms of diagnosis and problems with the prevalence of disease, the incidence of bladder cancer continues to be quite high for men and is actually the fourth most common cancer amongst men.
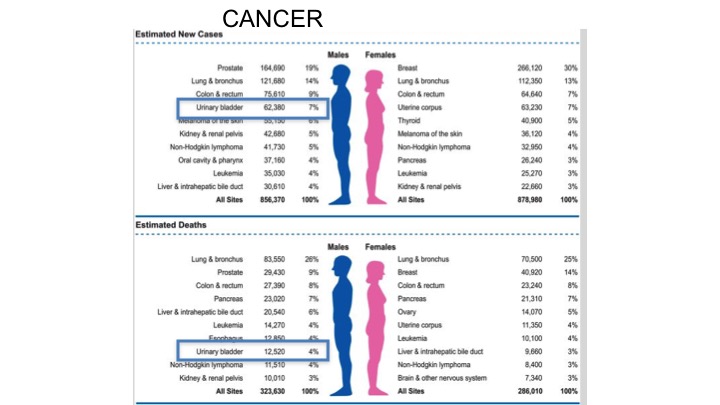
Cancer Statistics 2018
If you look at the stats for 2018, really not much has changed in terms of the number of men and women diagnosed with bladder cancer, and if you look at the curves for women the incidence in terms of bladder cancer doesn’t make the top ten, yet for men continues to be quite high. 7% in terms of urinary bladder there, and estimated deaths within the top ten again for bladder cancer.
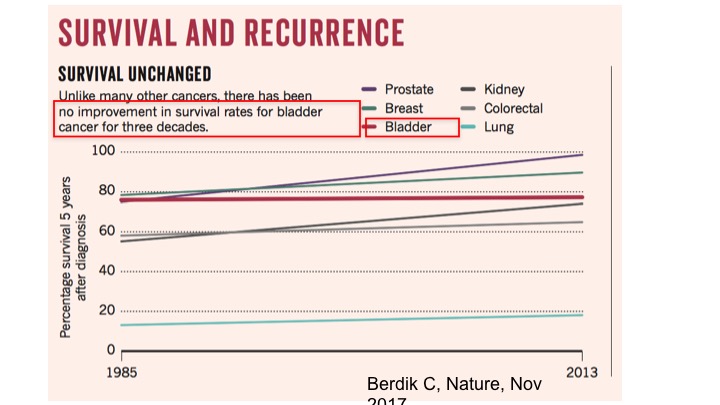
Survival and Recurrence
Unfortunately, though if you look at survival curves, really there has been no improvement in survival rates then for bladder cancer for the past three decades, whereas other types of cancers have shown a significant improvements. And so it is an attempt now, with the new actually treatment options that we have now available that we will talk about this afternoon, that hopefully, the survival curve will start changing.
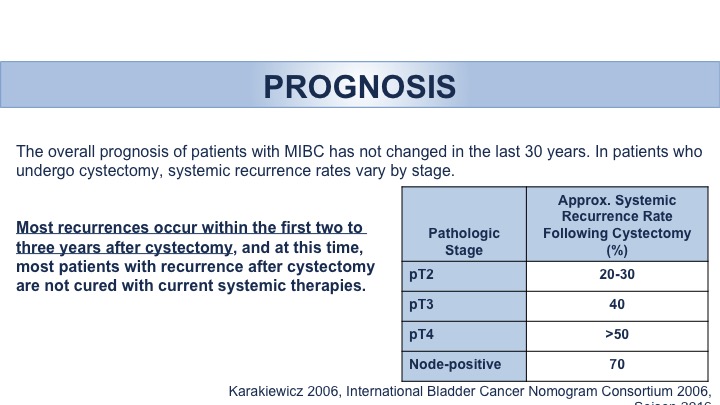
Prognosis: Recurrence
So the guidelines, this is a brief run through in terms of important points and points that I think I want to highlight. We had a discussion actually just this morning regarding the follow-up and surveillance of these patients. We know that for these patients then with invasive disease the occurrences tend to happen within the first two to three years, and as stage progresses in other words as your stage becomes higher and worse, the more dangerous your disease is, the more likely you are to have recurrence.
Prognosis: Survival Rates
In looking at pathologic features that are most important obviously pathology rules the actually outcomes in terms of not only recurrence and survival but other factors come into play as well, including hydronephrosis, lymphovascular invasion, as well as soft tissue margins and molecular subtyping, which we’ll again also talk about this afternoon.
Guideline Statements: Initial Evaluation And Counseling
So this is going to be a listing of the different types of guidelines, and I’ve underlined some points and I’ve actually bolded some points in red that I think are the most important. The guidelines need to have an initial evaluation, HNP, and those types of things, but for points that are important the staging evaluation should have some form of cross-sectional imaging, be it a CT scan or an MRI scan, have some type of baseline serum tests, the CMP and CBC and then the importance throughout the guidelines is a combination of understanding the importance of not only surgical options, but medical options and the medical options in the combination of radiation therapy as well as a stand point, and then the understanding that the patient is an important part of the decision making process.
Guideline Statements: Treatment: Chemotherapy (Nac/Ac)
So in understanding the importance of chemotherapy, level 1 evidence, and a point that was emphasized within the guidelines was the use of neoadjuvant, cisplatin-based chemotherapy, understanding that the cystectomy should occur within as soon as possible so that hopefully within an 8-week time period following chemotherapy to proceed with that cystectomy with combination therapy. I think Dr. Kamat made an excellent point this morning regarding that patient. If that point was found to have instead of T1 disease or actually T2 disease, then the importance and the evidence would suggest that neoadjuvant chemotherapy prior to cystectomy would be just beneficial for that patient in terms of survival outcomes. The points here in red are also important in terms of—there are now no validated that will-ell us at this hits point yet routinely whether or not certain indicators in terms of neoadjuvant chemotherapy are nor less likely to work. The best regimen and the duration of that therapy is still unclear and then understanding that not everybody can get neoadjuvant chemotherapy. Importantly the emphasis of Cisplatin was paramount, in other giving bad chemotherapy is worse than giving no chemotherapy at all. Now, now long you give it in terms of the specific agents, in other words gemcitabine, cisplatin, versus MVAC, versus dose and dense MVAC, we do not advocate any one other than the fact that cisplatin should be the treatment of choice.
Guideline Statements: Treatment: Radical Cystectomy
In terms of cystectomy, it’s a combination of not only removal in the males of the bladder and prostate seminal vesicles, and in females the bladder, uterus, and other GYN organs, but the combination of that plus a pelvic lymph node dissection. Importantly these guidelines, never done before, in terms of treatment options is understanding and making sure that the patients are counseled in regards to the sexual impact in terms of sexual function, and the fact that there certain organ preserving procedures that can be begun at the time of cystectomy for these patients.
Guideline Statements: Treatment: Urinary Diversion
Diversion—so in looking at the different types of diversion types, so ileal conduit versus orthotopic neo-bladder, versus – – diversions, clearly not one is superior to any other. It’s important that the combination of not only quality of life issues but the importance of what the possible side effects are associated with each of those diversion types should be discussed with the patient understanding that each diversion type has its own unique set of complications and possible impact on quality of life. Looking at the wide variety of studies out there in terms of what may or may not be beneficial is really dependent upon that individual patient, and there is no study that says quality of life is better for those type of diversion versus another type of diversion. Importantly, point 14 here I didn’t underline was the importance at the time of an orthotopic diversion to make sure a urethral margin is in fact negative.
Guideline Statements: Treatment: Perioperative Management
Perioperative management—Dr. Black actually will give a talk on pathways and enhanced recovery after surgery in terms of what is best for cystectomy patients. Importantly these guidelines actually give some evidence in regards to what can be helpful, what’s useful, and what should actually physicians consider along with their patients. That would include the use of these enhanced recovery pathways, the importance of nutritional counseling, we had a whole session yesterday afternoon regarding the benefit of overall optimization of not only nutritional status but exercise status as well, smoking cessation and bowel preparation. Actually, we’ll have a talk also this morning regarding the possibility of thromboembolic complications, the avoidance of that and the importance at the time of cystectomy to take that into account. There’s increasing data that these patients especially as they get neoadjuvant chemotherapy are even more likely to have thromboembolic complications, and you throw in the possibility of cystectomy along with these patients and it’s important to keep that in mind.
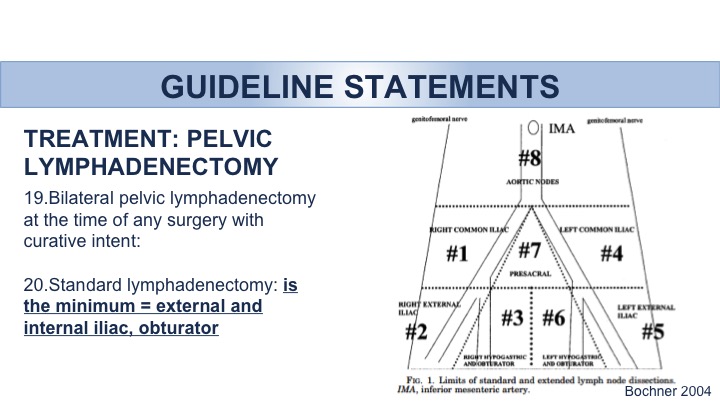
Guideline Statements: Treatment: Pelvic Lymphadenectomy
Dan, I’m sure that Peter will mention regarding alvimopan. In terms of the strength of evidence, this is clearly the one agent or intervention that has the highest quality of evidence in terms of decreasing the length of stay and decreasing any bowel complications associated with those patients undergoing radical cystectomy. Clearly, an impact of 1 to 2 days in terms of length of stay.
Big debate regarding pelvic lymphadenectomy. Two large trials have not yet reported final results regarding the ultimate benefit and/or perhaps lack of benefit with an extended lymph node dissection versus a standard lymph node dissection. At the very minimum it’s outlined that the external and internal iliac as well as the obturator lymph nodes be removed. The evidence within the guidelines suggests the possible benefit of a wider lymph node dissection vis-à-vis the possible side effects associated with that. But at the very minimum clearly lymph nodes need to be removed. A minimum standard lymph node dissection needs to be done because there is an impact on overall survival. Those patients that don’t have a lymph node dissection are less likely to live compared to those that actually do.
Guideline Statements: Bladder Preservation: Patient Selection
Patient selection, so importantly actually there is a patient – – was actually on the guidelines panel importantly emphasizing the impact of the fact that patients should have a clear role in choosing the therapy that they have for this particular issue and that bladder preserving therapy although not ideal for every patient should at least be discussed for every patient, and clearly it is the combination of a multi-modality bladder preserving strategy, so a combination of maximal TURBT, chemotherapy, as well as radiation therapy and continued follow up with the bladder is the optimal approach if bladder preservation is to be chosen, and that combination of therapy is far superior than any individual modality itself in terms of bladder preservation. Importantly that patient selection takes into account what the patient actually feels in terms of what may be in his or her best interests.
Guideline Statements: Maximal TURBT, Partial Cystectomy, and Primary Radiation Therapy
Looking at other possible bladder-preserving therapies, a combination of partial cystectomy alone versus a maximal TURBT should not be the primary therapy. In addition radiation therapy alone should not be offered as curative treatment. And remember these are actually guidelines supported by ASTRO as well. Clearly, the use of just radiation in and of itself should not be used as a curative treatment, can be used for palliation but in no way should be advocated as the treatment of choice for cure.
Guideline Statements: Multimodal Bladder Preserving Therapy
Here is a point regarding multi-modality therapy in terms of the chemotherapy agents out there. We talked about cisplatin-based neoadjuvant chemotherapy is the standard of care, and the treatment of choice for neoadjuvant chemotherapy. For those patients then who want bladder preservation therapy and what the best possibility of cures with multi-modality therapy, the chemotherapy options would include cisplatin or a combination of 5FU and Mitomycin. Importantly these patients will continue to need to have their bladders surveyed and evaluated not only through the process of actually getting those treatments but in follow-up afterwards, and in those patients that afterwards have disease that recurs there is within our treatment algorithm a decision tree that is important regarding those patients that actually have invasive disease, or high-risk disease. They should actually in fact undergo cystectomy. In those patients, however, with non-muscle invasive disease, or lower risk disease, then standard therapies actually can apply.
Guideline Statements: Bladder Preserving Treatment Failure
Treatment failure, we’ve talked about surgical treatment, and I just talked about the treatment in terms of conventional means for non-muscle invasive disease. Surveillance and follow-up there is no level 1 evidence saying that this treatment surveillance protocol is better than any other. But importantly we tried to take into account that remember the disease recurrence and/or new disease may occur usually within the first few years and as a result it’s recommended that every 6 to 12 months that a combination of imaging as well as an evaluation of the patient by laboratory assessment be done, and it’s a combination not only for evaluation for disease recurrence, but also for the impact that any urinary diversion has on metabolic symptoms or derangements that the patients may develop, and so for those reasons a combination of disease follow-up as well as overall outcomes after the diversion, a combination of not only imaging but serum studies are recommended, and importantly Dr. Lerner talked about evaluation of the urethra and how it should be done, what should be done, there is no specific outline saying one therapy or evaluation process is better than the other, but it is important that the urethra not only incontinent diversions but in ileal conduit diversions as well as continent cutaneous diversions that the urethra be monitored.
Guideline Statements: Patient Surveillance & Follow Up
Importantly we included different types of support groups and counseling that is available to the patients and wanted to make sure that was available for all of those out there, and then there is a whole section regarding the positive impact of not only health basically lifestyle choices but as well including the importance of nutritional choices as well as exercise choices within the guidelines, and the important of actually emphasizing that to the patient. Also, within the guidelines there is the impact of the possibility of the negative effects of malnutrition and actually the sarcopenia that was mentioned yesterday as well.
Variant Histology
Variant histology—we mentioned it briefly in the panel this morning, and actually I think Dr. Kamat may have a talk later on today about variant histologies as well. What we know is we don’t know a lot yet about variant histologies. There is a concern that these histologies definitely have a more aggressive nature, but regarding the best in optimal therapy for these, we yet do not know, and as a result for those individual patients these guidelines regarding the importance of neoadjuvant chemotherapy, the importance of combination therapy may not apply, and within the guidelines, there are certain disease characteristics where right now we don’t know if neoadjuvant chemotherapy is in fact beneficial at all, and in fact the recommendation is to proceed with radical cystectomy and removal of the bladder. So it’s in these patients we emphasize the importance of recognizing the variant histologies. Unfortunately we don’t know yet the optimal therapeutic intervention for these patients.
Future Research
Future research—I mean the emphasis on a combination of all the things we just talked about, and actually a lot will be discussed today as well, markers, evaluation in terms of follow up for these patients. We all mentioned in the panel that we would get urine cytology. Well, the impact and the beneficial impact of cytology after urinary diversion is really not known. The changes that can occur after a conduit diversion versus a conduit diversion are yet not known so a better marker for not only follow up and detection but for surveillance. The importance of optimizing further therapies, you saw the survival curves showing no difference whatsoever the past few decades. We hope now with new agents that we have now available that that curve will start to show an improvement and perhaps those interventions can occur sooner as opposed to later, and then the importance of surveillance. For the non-muscle-invasive guidelines, we’ve talked about actually decreasing surveillance for low-risk patients. That may be these patients as well at the time of bladder removal, they may end up being PT0 after neoadjuvant chemotherapy, so should those patients be monitored any differently? We yet do not know. So a lot yet continues to be looked at in terms of research.
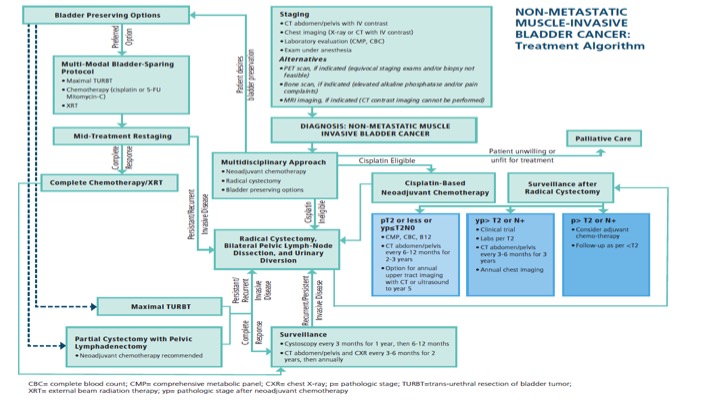
Non-Metastatic Muscle-Invasive Bladder Cancer: Treatment Algorithm
So this is a busy slide that shows the actual one-page dictum put out by AUA and ASCO and ASTRO to try to put on one page all of the different types of treatment modalities for those patients that have non-metastatic muscle-invasive bladder cancer, and as you go through the algorithm there are actually changes for if patients actually have positive margins, or higher risk disease, and/or recurrence, and what to do. So it’s an attempt to basically then codify on a page those patients in terms of best therapy options.
ABOUT THE AUTHOR
Dr. Chang is the Patricia and Rodes Hart Endowed Professor of Urologic Surgery and Oncology and the Oncology Fellowship Director and Vice Chair of Urologic Surgery at Vanderbilt University Medical Center in Nashville, Tennessee. He is a graduate of Princeton University and Vanderbilt University Medical School (where he earned a full 4-year Justin Potter Scholarship) and he completed his Uro-Oncology Fellowship as Chief Fellow at Memorial Sloan-Kettering Cancer Center. He also completed his MBA at Vanderbilt’s Owen School of Business.
Since his return to Nashville, Dr. Chang has focused on urologic oncology and education and has led efforts in the integration of evidence-based medicine in clinical pathways, enhanced national guidelines formulation, and improved urologic cancer staging. He has orchestrated the initiation and expansion of multiple cancer-related treatment protocols at Vanderbilt and elsewhere.
Dr. Chang has served as the Chairman of the SUO Panel on Hormone Refractory Prostate Cancer, the Chair of the American Joint Committee on Cancer GU Staging Task Force, the Chair of the AUA/SUO Guidelines on Noninvasive Bladder Cancer, the Facilitator and Vice-Chair of the Renal Malignancy Follow-Up AUA Guidelines Panel, the Chair of the AUA/ASCO/ASTRO/SUO Guidelines Panel on Non-Metastatic Invasive Bladder Cancer, and the Chairman of the AUA Prostate Cancer Core Curriculum Committee. He is a former member of the NCCN Kidney Cancer Panel. In addition, he was an AUA-EAU Academic Exchange Fellow.
Dr. Chang has authored more than 250 original publications, multiple book chapters and edited several textbooks. For his academic efforts, he received the SUO’s first-ever Distinguished Service Award, a CaP CURE Young Investigator Award, and has been named multiple times as a Journal of Urology’s Best Reviewer.
Dr. Chang was named as the 2011 recipient of the American Urologic Association Gold Cystoscope Award and completed a term as a nominated Fellow of the Nashville Health Care Council in 2016.