How to cite: Theodorescu, Dan. “Basic Science Foundations of Bladder Cancer Diagnosis and Treatment” January 27, 2018. Accessed Dec 2024. https://dev.grandroundsinurology.com/Basic-Science-Foundations-of-Bladder-Cancer-Diagnosis-and-Treatment/
Summary:
Dan Theodorescu, MD, PhD, describes clinically relevant scientific concepts underlying bladder cancer biology and the technologies, including biomarkers, Polymerase Chain Reaction (PRC), and Western blots, that have enabled the formation of our knowledge today. He then explains how these concepts have and will be used in the clinical management of bladder cancer.
(Twitter Question) Reveal the Answer to Audience Response Question #1
Finish the sentence- Cancer is driven by:
- A. Uncontrolled meiosis
- B. Uncontrolled mitosis
- C. Cell rupturing and death
- D. Low “Warburg effect”
- E. None of the above
Reveal the Answer to Audience Response Question #2
(Choose all that apply)
- A. Pre-emptive Cellular Reaction.
- B. Polymerase Chain Reaction.
- C. Detects cancer cell growth and reactivity to therapy.
- D. Amplifies specific DNA in a cell.
- E. Amplifies specific proteins in a cell.
Reveal the Answer to Audience Response Question #3
Finish the sentence- “SNP” (pronounced “SNIP”) is:
(Choose all that apply)
- A. A new vasectomy technique.
- B. Superior Negative Probability of a diagnostic test.
- C. Detects differences in DNA sequences between people.
- D. Single Nucleotide Polymorphism.
- E. Detects differences in RNA sequences between cancer cells.
(Twitter Question) Reveal the Answer to Audience Response Question #4
Finish the sentence- The Western Blot:
(Choose all that apply)
- A. Is the foundational technology that ELISA is based on.
- B. Is a molecular technique developed in Colorado.
- C. Cannot be used on animal tissues.
- D. Detects DNA in a cancer cell.
- E. Is used to detect specific proteins in a cell.
Basic Science Foundations of Bladder Cancer Diagnosis and Treatment – Transcript
Click on slide to expand
Lecture Objectives
Basically, what I would like to do is go over some of the principles and basic science that area really underpinning, the biology and the biomarkers, and many of the findings that you’ve heard throughout the last day or so in bladder and of course in prostate cancer as well. These things are very applicable. So these are fundamental ideas and procedures that have been used to discover the scientific findings underlying almost everything you’ve heard about so far today.
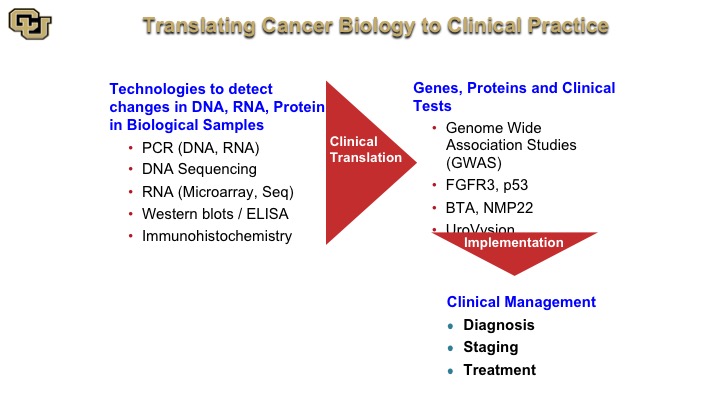
Translating Cancer Biology to Clinical Practice
So the way this works is you take technologies and they are listed here such as PCR, DNA, RNA, microarrays, Western blots, and I’m going to go and explain these, and these are basically used to identify the function and the relevance of genes and proteins such as FGFR3, P53, and lead to test such as the BTA and NMP22, and again as you heard earlier from Peter Black, these are not the be-all-and-end-all tests. But I want to show you, paradigmatically, how these were discovered, because many of the tests that do work are based on protein and DNA technology. And these of course are used in diagnosis, staging and treatment.
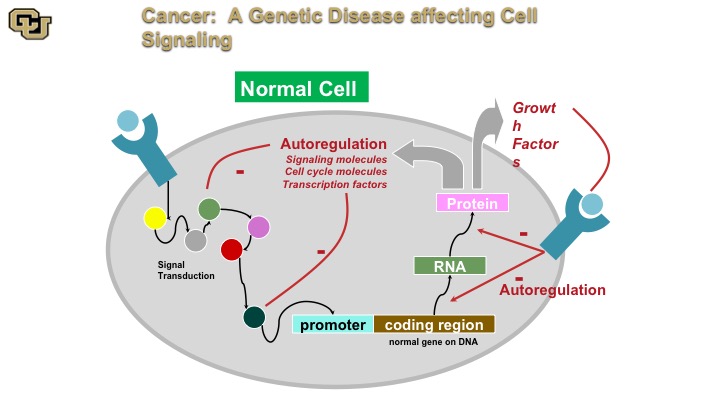
Cancer: A genetic disease affecting cell signaling
Cancer is a genetic disease. And basically this is a normal cell. A normal cell really has signal transduction pathways, basically the cellular machinery inside the cell that transmits signals as you can see from some of the outside receptors on the surface throughout the cell via various proteins, and then on to a promoter, and then the promoter regulates gene expression, which makes an RNA protein that then gets put out of the cell or inside the cell with auto-regulation internally and this is basically how the cell auto regulates itself through positive and negative feedback.
Cancer: A genetic disease affecting cell signaling Part 2
On the other hand the cancer cell doesn’t have those negative feedback loops so as a result of mutations in various of these components the components the cancer cell no longer can stop positive signals from the outside or from the inside, such as oncogene, and I’ll show you that in a minute to keep going and therefore leads to uncontrolled cell division called mitosis.
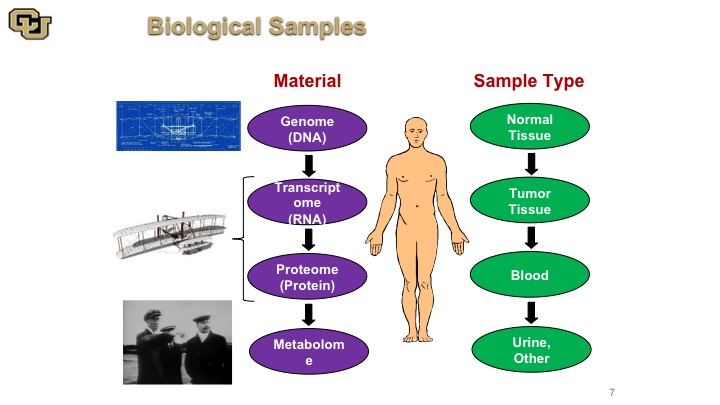
Biological Samples
So there are many things that we could use as reagents really to discover how cancer works, and there are many materials and I think of it the analogy with the Wright brothers’ plane. You have the blueprint which is the DNA. You have the RNA and the protein which is basically the model of the plane, and then you have the metabolome, which is basically the plane actually flying. So all of these things are for complementary aspects of understanding cancer. None of them are mutually exclusive, and depending on what you want to find or what you what you want to use for a diagnostic or prognostic factor or other target of therapy, you need different parts of this. So obviously those are the materials, and the sample types are also varied. You use normal tissue, tumor tissue, and body fluid, such as blood and urine.
Technologies Evaluate Samples—Biomarkers
So the next thing that you can sort of conceptualize is the following so when you do those analyses on the samples that I just showed you in the previous slide, you can do a number of things so germ line and host evaluation, that basically looks at the person that has the cancer. And that could be looked at using multiple techniques, microarray based or sequencing based, and then you could look at the tumor and body fluid and this has been and you have seen many of these examples throughout the last few days by various technologies, RNA expression, mutation analysis, copy number, IHC, immunohistochemistry for protein, and then immune checkpoint infiltrates you just heard from Ashish about some of these markers and Peter Black as well. So you take all of this and then you basically come together with an integrated analysis that gives you a molecular blueprint between the tumor and the host giving you a lot of information about this. And in discovery studies these technologies can be used for predisposition marker development, prognostic marker, predictive marker, and the predictive markers in many cases are targeted targets for drug development,. So these are some of the things I’m going to show you in the next few slides that– examples of how this technology was used to develop these for bladder cancer. And of course we hope that these are useful in clinical management and importantly this sort of process is reiterative, so when you have a tumor recurrence you repeat the process to profile the tumor to see if—what markers come out.
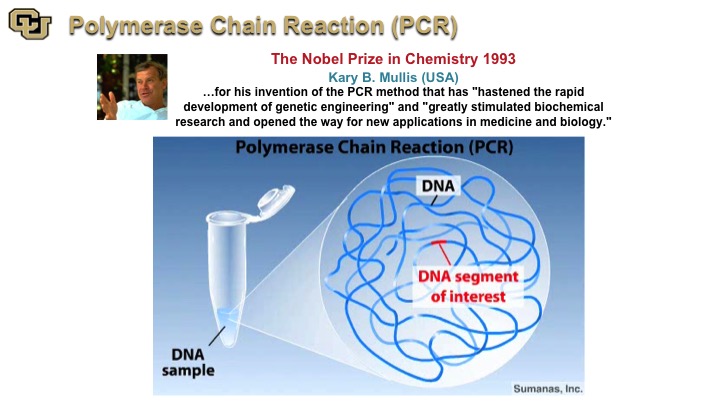
Polymerase Chain Reaction (PCR)
PCR is one of the most—it’s hard to say you know, there are Nobel prizes, and there are Nobel prizes, so this is one of the Nobel prizes which is Nobel prize that has enormous, immense, fundamental and clinical application because it is basically the root of almost everything that we do in genomic medicine currently so it has had transformative, beyond transformative advance. So I’m going to show you a little video of how this really works. It’s incredibly clever and shows fundamentally what you can do when you actually do fundamental science with no possible clinical impact. Kary Mullis basically made a fundamental observation from putting two very basic things together. Number one, he looked at bacteria that thrive in very hot environments that have certain RNA polymerases, and then looked at replication of another organism that replicates its DNA and combined those two features to be able to vary the temperature and by varying the temperature using cycles, from hot to cold, hot to cold, amplify DNA. He thought of it as a basic science finding with no clinical application, but obviously amplifying DNA could have major impact, and it has had incredible impact. So here you go.
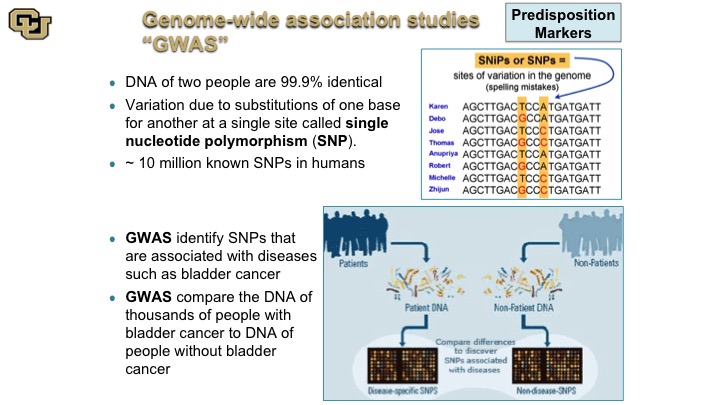
Genome-wide association studies “GWAS”
So the SNP is really the difference of single nucleotide polymorphism is really at the core of GWAS studies, which some of you may have heard of, genome wide association studies. So basically what these are, as is shown in this cartoon here, people are different with the—they are basically different SNPs, and these are just a hypothetical example and what these studies do essentially is they look at the differences between DNA sequence among individuals between patients say that have bladder cancer and patients that do not, and of course there are other differences between those two patient populations, smoking etc. but those are adjusted for and then the idea is to discover if there are germ line changes, so basically germ line meaning DNA changes in the DNA that makes the person that predispose you to bladder cancer.
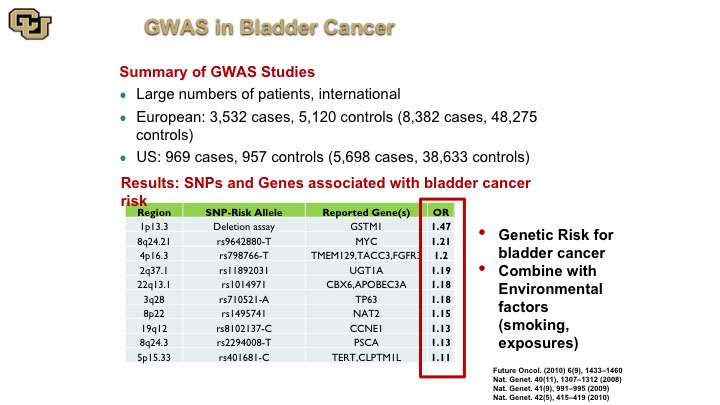
GWAS in Bladder Cancer
So here is a complication of many of the GWAS studies that were done bladder cancer, and there’s a website that does these compilations, and these are the alleles basically the piece of DNA that is different among people, and this is the gene that is next to it or implicated in that change, and this is the risk, the increased risk if you will of getting bladder cancer if you have that specific DNA mutation and just a point of note is just because you have that that doesn’t mean that there is not a lot of work to be done to figure out what the mechanism is between basically having that change and why you get bladder cancer. That does not come with the GWAS study. There’s a lot more work that needs to be done to figure that out. This is purely an association, and you can see that there are differences here of specific alleles and SNPs that increase your risk of bladder cancer. Unfortunately, the reality of the matter is that especially when you correct for the risk factors these things do not allow you to identify high-risk groups of patients based on germline changes so nevertheless this has been more successfully used in other cancers.
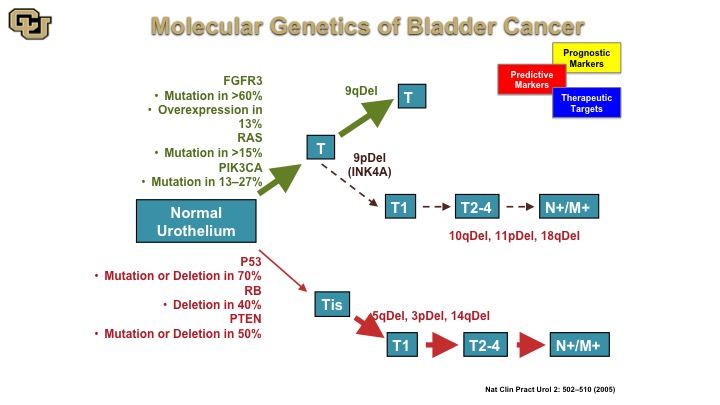
Molecular Genetics of Bladder Cancer
So the other thing I wanted to go over with is basically how the molecular biology of bladder cancers can be used for diagnostics and therapy and this is a cartoon that has been worked on and enhanced over the years, but the fundamentals of this are still true to this day although some of these ideas started in the mid-90s that essentially there are two pathways towards bladder cancer development, one that is mediated by the FGFR3 RAS, PI3 kinase, and another one that is P53 REMEMBER PTEN to leading to muscle invasive disease whereas the previous one is essentially non muscle invasive disease. Again, there are lot of generalities I’m making here to make the points that you will see in the next few slides.
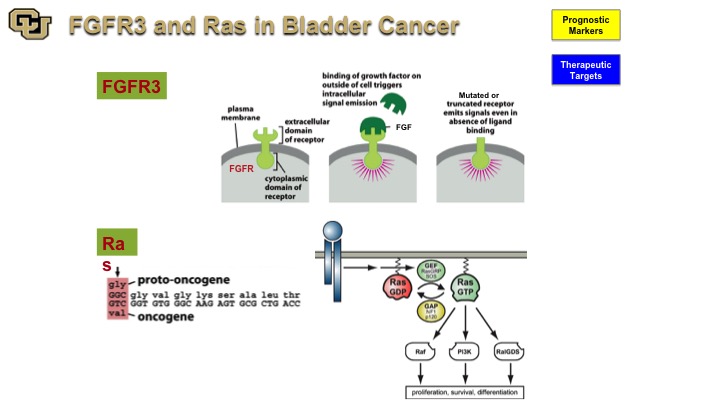
FGFR3 and Ras in Bladder Cancer
So the two genes that have been implicated with a non-muscle invasive disease are FGFR3 and Ras both of which have mutations with a high frequency in bladder cancer. And you’ve seen from Peter Black‘s talk earlier that those are some of the genes that are found in the mutation-based tests for detection of bladder cancer because they are very commonly found and mutated in bladder cancer. Both are signal transduction molecules, one’s a receptor, and one is an intracellular molecule. FGFR3 is particularly interesting because it was not discovered as a cancer gene. It was actually found to be a cause for achondroplasia, causing fusion of the bones prematurely. In cancer, of course, it leads to uncontrolled firing of the FGFR3 receptor when it is mutated leading to proliferation and growth.
FGFR3 Mutations Associated with Improved Prognosis in NMI Bladder Cancer
The interesting thing about it is that many studies have shown that the mutation is actually associated with a better outcome of patients with non-muscle invasive bladder cancer. I’m not going to go into these studies, but I’ve listed them here to make the point that this has been used, this has been generally associated with a better prognosis disease.
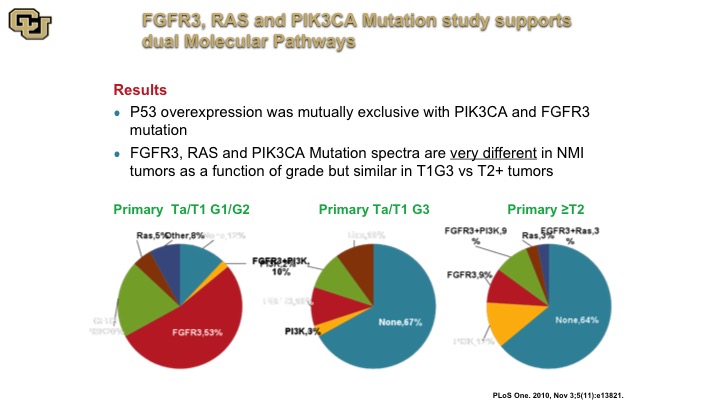
FGFFR3, RAS and PIK34CA Mutation Study Supports Dual Molecular Pathways
The other thing that came out of these studies that was very interesting was the fact that they were demonstrating for the first time the presence of these two molecular pathways, the FGFR3, the RAS, versus the P53 so you could see this by the various pie charts. This is a primary tumor that was low grade essentially and this is a tumor that was primary but high grade, and you can see they have very different profiles in these non-muscle invasive associated mutant genes, but the primary tumors that were muscle invasive looked a lot more like the high grade non-muscle invasive disease suggesting in fact they arose from that sub-group of cells. So the point is that high-grade non-muscle invasive disease is not a good thing, and is more like molecularly to a muscle invasive tumor, you just happen to have caught it early.
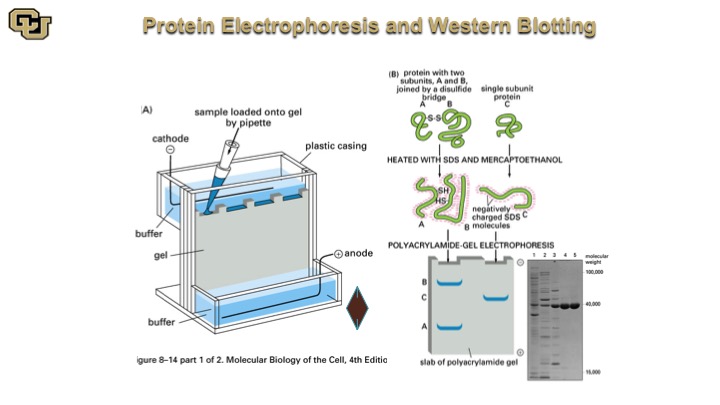
Protein Electrophoresis and Western Blotting
So this is a Western Blot here, so you do essentially a protein extraction, and you denature the proteins, and then you pipette them down this electrophoresis gel and then the proteins separate based on migration in this gel and they look like bands like this after the gel is stained with a specific antibody and you quantitate the amount of protein that the antibody detects.
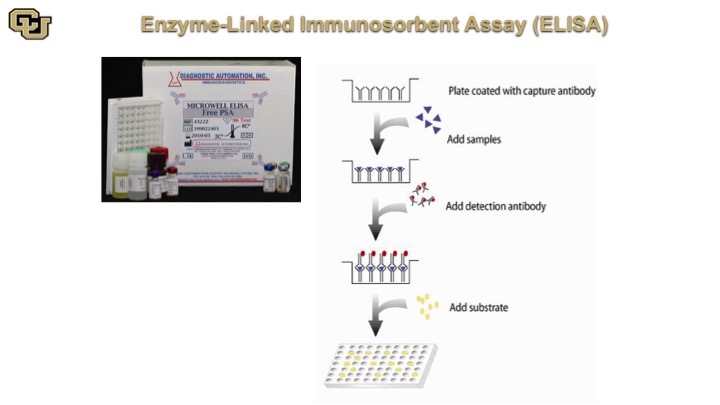
Enzyme-Linked Immunosorbent Assay (ELISA)
This is very similar in concept and matter of fact the ELISA is based on the Western because the ELISA is an antibody that is at the bottom of the plate, you add your sample, and if you are looking for say PSA the PSA is going to bind to antibodies on the bottom and then you come back with the same antibody that is labeled and it will detect basically the PSA molecules bound on the bottom antibody. So essentially it’s in the Western double capture Western in a well.
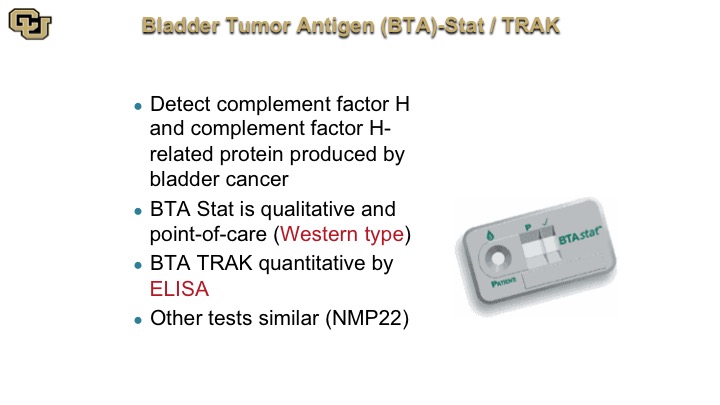
Bladder Tumor Antigen (BTA)-Stat/TRAK
So this technology is the underlying technology that is underlying the BTA STAT and BTA TRAK.
Fluorescence in Situ Hybridization (FISH)
Another development that is unrelated to the Western but moving on is the FISH technology. So Fluorescent in situ hybridization underlies another technology of detection of bladder cancer, UroVysion I’ll show you that in a minute. What this does is basically have fluorescent DNA probes that detect chromosomal abnormalities which are common in cancer and detects specific genetic changes in a chancer cell. So here is sort of a cartoon describing this, the UroVysion and so it has several fluorescent probes looking at the centromere on chromosome 3, 7, and 17, which are often abnormal in bladder cancer cells, and then it has locus specific probes at specific spots on the chromosome that are lost in cancer or gained in cancer, so you show—you apply these fluorescent probes, and then the slides are looked at just like a cytology by a human and then scored basically seeing if you have more than two copies. Obviously if you have more than two copies, say pink here for example, in this nucleus, that is obviously a cancer cell because you are not supposed to have more than two copies in a normal cell. So that is the principle of the UroVysion that you’ve heard about earlier.
p53 in Bladder Cancer
Now, let me move on to p53, and this is one that is sort of the poster child of mutations. p53 is a probably the most studied gene in cancer. If it is not the most, it is close, it is up there, and it’s usually altered by a point mutation. There are a lot of point mutations in p53, and many of these result in extended half life of the protein and that extended half-life is actually detectable by immunohistochemistry, which I will show you and of course the mutation is detected by DNA sequencing.
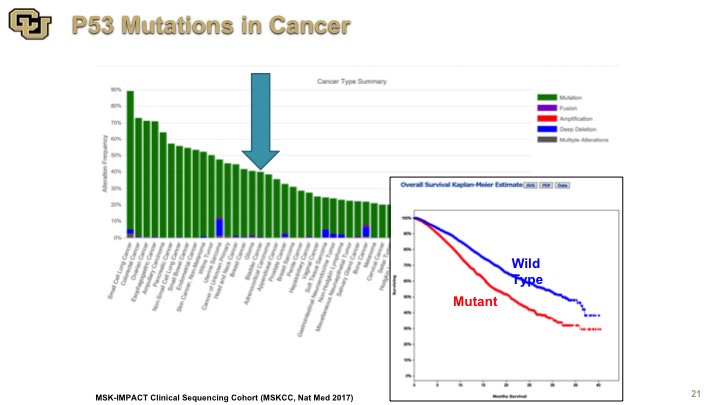
p53 Mutations in Cancer
So this is an example of the data from the IMPACT study which was done out of Memorial Sloan Kettering and they’ve sequenced to almost 10,000 patients for a variety of genes, but this is the data on p53, and this is the Kaplan-Meier curve of survival. Again, you see the number of cancers here, and some are hidden by this other chart. There are lot, but a lot or not, the point is that mutant p53 is not good for you and of course it is a very heterogeneous group many types of cancers, many different treatments, nevertheless p53 is not good for you, so mutation is not good for you.
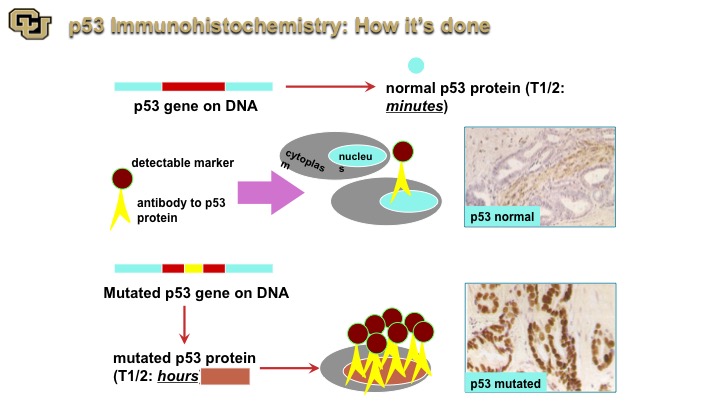
p53 Immunohistochemistry: How its done
So that coupled with immunohistochemistry and now I’m going to focus more on bladder cancer, immunohistochemistry really if you will is like a Western except on a tissue slice. You go in with an antibody that goes with the protein that detects the protein and you can see p53 normal, p53 that is not mutated generally has a very short half-life. When it’s mutated it has a longer half-life and you can detect it. This is an example of p53 mutated, very dark nuclei compared to light brown.
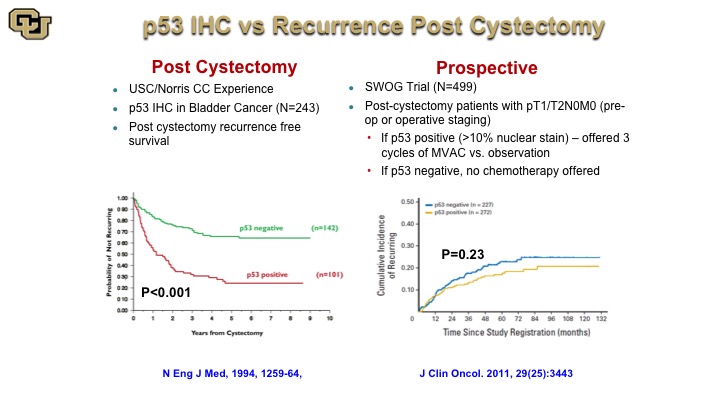
p53 IHC vs. Recurrence Post Cystectomy
This is a very old study from John Stein and Don Skinner showing post cystectomy stratification based on p53 negative or positive by immunohistochemistry but subsequent studies and a large trial that was based on this finding that then assigned patients to chemotherapy based on P53 status, unfortunately did not reveal any difference in outcome, which was a great disappointment especially that this trial took about 10 years to accrue to. Nevertheless it highlights the fact that when you look at a protein at the immunohistochemistry level for its presence or absence that loses a lot of resolution in terms of the refinements on the mutations because the mutations occur throughout the protein, so not every mutation is the same, has obviously the same biological impact.
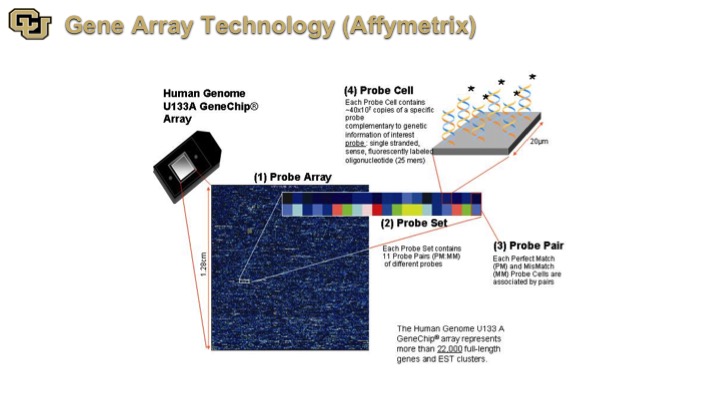
Gene Array Technology (Affymetrix)
So finally some of the later technologies are Affymetrix, and what this is is basically a detection of genes on a very large scale, essentially looking at all of the genes in the genome and the immediately application for this is for biomarkers.
GeneArray Clinical Use
This is an Affymetrix examination across multiple tumor types, and the investigators identify a cluster of genes that are expressed on specific tumors, and this is now commercially used to identify the original histological source from tumors of unknown origin, so patients that present with metastatic tumor and it’s not obvious where the primary is.
DNA: Next Generation Sequencing (NGS)
The next generation sequencing, obviously, has been a landmark advance in cancer in general and many papers have focused on bladder cancer in the past 5 or so years, and this has lead not only to biomarkers of detection and diagnostics but also targeted therapy. I’m going to show you one final example now of how a mutant protein has been used successfully, probably the poster child of targeted therapy that, actually I hate to use the word “cures,” but leads to very, very, very prolonged remission in a cancer.
Cancer Cell Mutations as Drug Targets
That is basically Gleevec, and the basis of that is as I mentioned a minute ago a drug that really attacks mutant proteins that are destabilizing really the signaling cascade in a cancer.
Bladder Cancer
Basically that concludes my talk. Obviously we live in an incredible time in cancer biology, really at the tipping point of you know I think changing the natural history of many, many different cancers with immunotherapy with targeted agents and combinations thereof. So it’s a fantastic time, and I think many of us really feel privileged to feel part of it. So thank you.
ABOUT THE AUTHOR
Dr. Theodorescu is the Director of Samuel Oschin Comprehensive Cancer Institute at Cedars-Sinai and former Director of the University of Colorado Comprehensive Cancer Center.
Working on bladder cancer, Dr. Theodorescu has emerged as a leading translational cancer researcher. His pioneering application of computational biology led to the discovery of genes that regulate tumor growth and metastasis (RhoGDI2, AGL, GON4L) in bladder and other cancers, and novel biomarkers and concepts for precision therapeutic approaches such as the COXEN principle that are currently being tested in national (SWOG) clinical trials. He also conceptualized the approach and then led the discovery of a “first in class” RalGTPase inhibitor as a new therapeutic in several human cancer types that is currently in commercial development.
Dr. Theodorescu is both a project leader and co-program leader of an NCI program project and SPORE. He is the recipient of many awards, sits on several boards, and has been elected to prestigious societies including the American Society for Clinical Investigation (ASCI), the American Association of Genitourinary Surgeons (AAGUS), the American Surgical Association (ASA), and the National Academy of Medicine (NAM) (formerly Institute of Medicine (IOM) of the National Academy of Sciences). Theodorescu is the founding co-editor of Bladder Cancer, the first journal focused on this disease.
From 2010 to 2018, Dr. Theodorescu served as Director of the University of Colorado Comprehensive Cancer Center (UCCC), a statewide consortium encompassing three universities and three academic teaching hospitals. He successfully led the center through two NCI Cancer Center Support Grant (CCSG P30) renewals, the most recent in June 2016 where the NCI review team stated that “Dr. Theodorescu has provided exceptional leadership and exemplary management of the UCCC, positioning the Cancer Center to continue in advancing its national role.” Over the past five years, this national role has been enhanced by election into the National Comprehensive Cancer (NCCN) and Oncology Research Information Exchange (ORIEN) Networks. The UCCC also rose from unranked (2010) to 15th (2015) cancer program in US News and World Report. His experience as a surgeon and in biotech, academic administration, and in interactions with elected officials has provided him with insights on how relationships between medical staff and academic, commercial, and governmental entities can be crafted to improve local, regional, and national health care. Emblematic of this was his work with Reps. Upton (R-MI) and DeGette (D-CO) on H.R. 6, The 21st Century Cures Act that was passed by the House in July 2015. He also secured State of Colorado funding for the Center in 2016.