Daniel P. Petrylak, MD, presented “Bladder Cancer and Immunotherapy” during the 3rd Annual International Bladder Cancer Update on January 23, 2019 in Beaver Creek, Colorado.
How to cite: Petrylak, Daniel P. “Bladder Cancer and Immunotherapy” January 23, 2019. Accessed Dec 2024. https://dev.grandroundsinurology.com/bladder-cancer-immunotherapy/
Bladder Cancer and Immunotherapy- Summary:
Daniel P. Petrylak, MD, reviews data regarding currently-approved immunotherapy agents for bladder cancer in first- and second-line settings. He advocates for future research regarding immunotherapy-based combinations, as well as investigating immunotherapy in earlier stages of the disease.
Abstract:
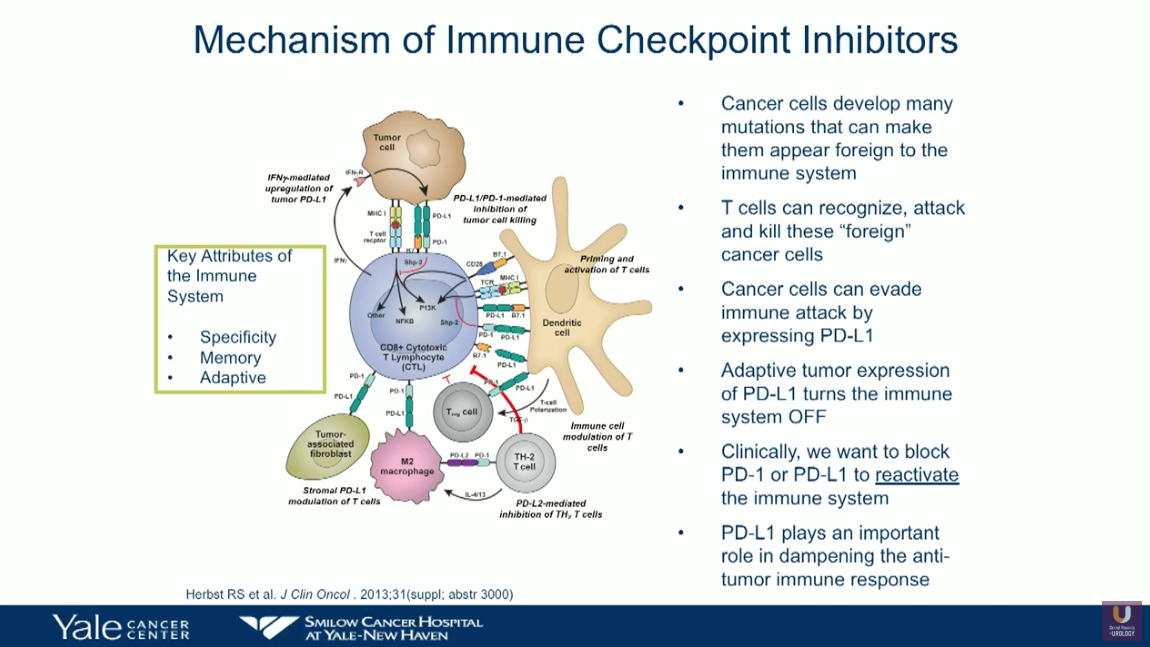
Immunotherapy has emerged as a promising treatment option for patients with bladder cancer. Of the five currently-approved checkpoint inhibitors for urothelial carcinoma (UC), atezolizumab and pembrolizumab have approval in the first-line, cisplatin-ineligible setting, as well as in the platinum-pretreated patients. Nivolumab, durvalumab, and avelumab only have approval for platinum-pretreated patients.
Patient selection for first-line checkpoint inhibition therapy remains a challenge due to inconsistencies in the definition of medically unfit patients. Nonetheless, the treatment goals for these patients has shifted towards palliation of symptoms rather than survival or therapy response. This presentation reviews findings from IMvigor210 (Cohort 1) and KEYNOTE-052 trials as they pertain to checkpoint inhibitors as first-line therapy for cisplatin-ineligible patients.
Results from these trials led to the exploration of potentially moving checkpoint inhibitors to the front-line cisplatin-eligible patients, as well as a restructuring of therapy sequencing for cisplatin-ineligible patients. This presentation analyzes recent data on response rates to checkpoint inhibition in patients with PD-L1 low status, as well as first-line chemotherapy/checkpoint inhibition combination in metastatic UC.
Future research on this topic should focus on the possibility of immunotherapy for non-muscle invasive disease, investigating further therapy combinations, and developing of biomarkers beyond PD-L. Additionally, physicians should maintain a thorough understanding of the markers of resistance and response, as this will help when designing future trials in earlier disease.
About the International Bladder Cancer Update
The International Bladder Cancer Update (IBCU) is an annual one-day CME conference focused on bladder cancer treatment updates. IBCU takes place during its sister conference, the International Prostate Cancer Update (IPCU). The conference’s faculty consists of international experts, and the event caters to urologists, urologic oncologists, and other healthcare professionals. In addition to didactic lectures, IBCU features interactive discussions, a panel roundtable, debates, and case presentations. Dr. Petrylak presented this lecture during the 3rd IBCU in 2019. Please visit this page in order to learn more about future IBCU meetings.
ABOUT THE AUTHOR
Daniel P. Petrylak, MD, is currently Director of Genitourinary Oncology, Professor of Medicine and Urology, Co-Leader of Cancer Signaling Networks, and Co-Director of the Signal Transduction Program at Yale University Cancer Center in New Haven, Connecticut. He is a recognized international leader in the urology field. He earned his MD at Case Western Reserve University School of Medicine in Cleveland Ohio. He then went on to complete his Internal Medicine Residency at Albert Einstein College of Medicine/Jacobi Medical Center in the Bronx, and his fellowship at Memorial Sloan Kettering Cancer Center in New York.
Dr. Petrylak has served as principal investigator (PI) or co-PI on several SWOG clinical trials for genitourinary cancers. Most notably, he served as the PI for a randomized trial that led to the FDA approval of docetaxel in hormone refractory prostate cancer. He also helped to design and served as PI for the SPARC trial, an international registration trial evaluating satraplatin as a second-line therapy for hormone refractory prostate cancer.
Dr. Petrylak served on the program committees for the annual meetings of the American Urological Association from 2003-2011, and for the American Society of Clinical Oncology from 1995-1997 and 2001-2003. He also has served as a committee member for the Devices and Immunologicals section of the FDA. He has published extensively in the New England Journal of Medicine, Journal of Clinical Oncology, Journal of the National Cancer Institute, Cancer Research, and Clinical Cancer Research.