Daniel P. Petrylak, MD, presented “Debate: Neoadjuvant Chemotherapy Prior to Cystectomy | Pro Argument” during the 27th Annual Perspectives in Urology: Point Counterpoint on November 9, 2018 in Scottsdale, Arizona.
How to cite: Petrylak, Daniel P. “Debate: Neoadjuvant Chemotherapy Prior to Cystectomy | Pro Argument” November 9, 2018. Accessed Jan 2025. https://dev.grandroundsinurology.com/debate-neoadjuvant-chemotherapy-prior-to-cystectomy-pro-argument/
Debate: Neoadjuvant Chemotherapy Prior to Cystectomy | Pro Argument – Summary:
Daniel P. Petrylak, MD, argues the advantages of using neoadjuvant chemotherapy prior to cystectomy to treat patients with muscle invasive bladder cancer (MIBC). He reviews evidence including the SWOG 87-10 trial and a meta-analysis to support this stance.
This presentation is the “pro” or positive installment of a debate over the use of neoadjuvant chemotherapy prior to cystectomy for MIBC. The rebuttal to this argument is “Debate: Neoadjuvant Chemotherapy Prior to Cystectomy | Con Argument” by Michael S. Cookson, MD, MMHC.
Evidence of Pro Stance: SWOG 87-10
Data from the SWOG 87-10 trial provides some evidence supporting the use of neoadjuvant chemotherapy prior to cystectomy in MIBC. The SWOG 87-10 trial is the first in this context to demonstrate a survival advantage in favor of chemotherapy. However, the regimen given in the SWOG 87-10 trial was relatively toxic. The rate of cytopenia was fairly high, but there were no reported toxic deaths related to chemotherapy. Also, regarding patients with more advanced disease, administering chemotherapy later on becomes difficult. Comparing post-cystectomy complications between the cystectomy alone arm and chemotherapy prior to cystectomy arm, there were no differences in hemorrhage, embolisms, edema, infection, and genitourinary events. These results support the safety of neoadjuvant chemotherapy.
The trial showed a 74-month median survival for patients receiving chemotherapy compared to the control arm, which had a 43.2-month median survival. Notably, neoadjuvant chemotherapy showed a survival benefit across all grades and stages, with the greatest observed benefit being in T3 disease.
Clinical Evidence to the Benefits of Neoadjuvant Chemotherapy
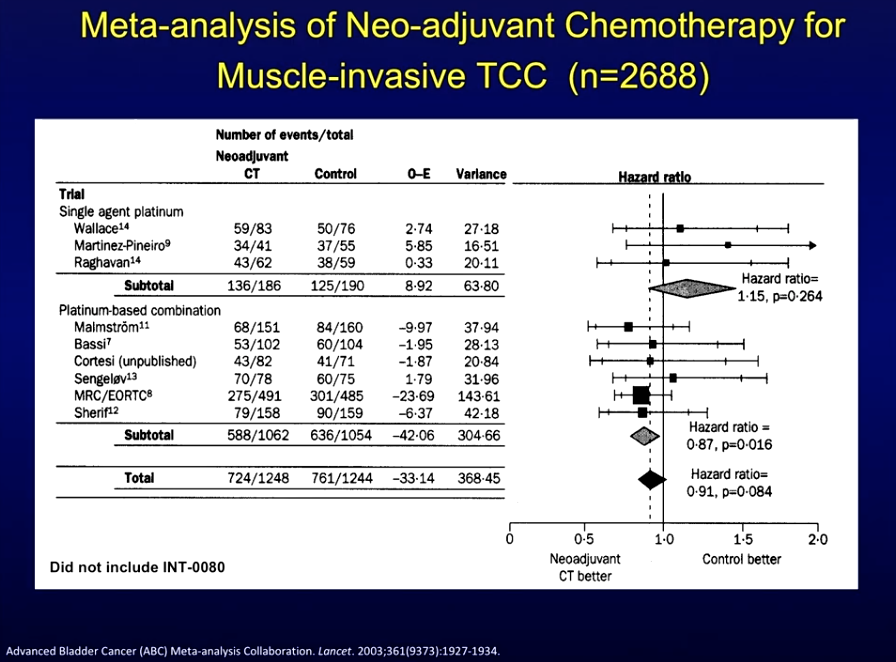
Corroborating these results, a meta-analysis of trials comparing neoadjuvant chemotherapy plus local treatment against the same local treatment alone showed results all generally favored neoadjuvant treatment.
New regimens of neoadjuvant chemotherapy are under investigation. For instance, data on a new dose-dense methotrexate, vinblastine, adriamycin, and cisplatin (MVAC) neoadjuvant chemotherapy regimen is promising. The National Comprehensive Cancer Network (NCCN) has accepted this regimen as a frontline therapy. Additionally, a study comparing MVAC, the mainstay of treatment in this setting, with gemcitabine and cisplatin (GC), showed no difference in overall outcomes between the two arms. As a result, the NCCN now accepts GC as an alternative regimen.
Neoadjuvant chemotherapy has a potential bladder-sparing benefit, however, there is controversy surrounding this possibility. Studies by Herr and Sternberg show about three-quarters of patients survive following bladder-sparing surgery,about half with their bladder intact, and that salvage chemotherapy is an option for these patients.
Arguably, neoadjuvant chemotherapy remains a superior option to adjuvant chemotherapy, as there is no study to date showing a proven survival benefit with adjuvant chemotherapy. While adjuvant chemotherapy allows for the selection of high-risk patients and avoidance of overtreatment, it does not allow for the option of bladder preservation, and may come with a burden of increased toxicity.
About Perspectives in Urology: Point Counterpoint
Perspectives in Urology: Point Counterpoint (PCP) is an annual CME-accredited conference devoted to discussing and debating the latest topics in men’s health, general urology, and genitourinary cancers. The conference’s format includes more than didactic lectures. It also includes debates, point-counterpoint discussion panels, and unique case-based presentations. Dr. Petrylak presented this lecture during the 27th PCP in 2018. Please visit this page in order to register for future PCP meetings.
ABOUT THE AUTHOR
Daniel P. Petrylak, MD, is currently Director of Genitourinary Oncology, Professor of Medicine and Urology, Co-Leader of Cancer Signaling Networks, and Co-Director of the Signal Transduction Program at Yale University Cancer Center in New Haven, Connecticut. He is a recognized international leader in the urology field. He earned his MD at Case Western Reserve University School of Medicine in Cleveland Ohio. He then went on to complete his Internal Medicine Residency at Albert Einstein College of Medicine/Jacobi Medical Center in the Bronx, and his fellowship at Memorial Sloan Kettering Cancer Center in New York.
Dr. Petrylak has served as principal investigator (PI) or co-PI on several SWOG clinical trials for genitourinary cancers. Most notably, he served as the PI for a randomized trial that led to the FDA approval of docetaxel in hormone refractory prostate cancer. He also helped to design and served as PI for the SPARC trial, an international registration trial evaluating satraplatin as a second-line therapy for hormone refractory prostate cancer.
Dr. Petrylak served on the program committees for the annual meetings of the American Urological Association from 2003-2011, and for the American Society of Clinical Oncology from 1995-1997 and 2001-2003. He also has served as a committee member for the Devices and Immunologicals section of the FDA. He has published extensively in the New England Journal of Medicine, Journal of Clinical Oncology, Journal of the National Cancer Institute, Cancer Research, and Clinical Cancer Research.