Seth Lerner, MD, presented “BCG Unresponsive Disease—A Roadmap for Drug Development and Integration of Novel Therapies” at the 2nd Annual International Bladder Cancer Update on January 27, 2018 in Beaver Creek, Colorado
How to cite: Lerner, Seth. “BCG Unresponsive Disease—A Roadmap for Drug Development and Integration of Novel Therapies” January 27, 2018. Accessed Dec 2024. https://dev.grandroundsinurology.com/Drug-Development-and-Integration-of-Novel-Therapies/
Summary:
Seth Lerner, MD, defines what places a bladder cancer patient into the BCG unresponsive category and treatment options in these cases. He touches on valrubicin, summarizes data regarding off-trial options such as gemcitabine, docetaxel, and the gem/doce combination, and discusses clinical trials for CG0070, Ad-IFN, and atezolizumab.
Reveal the Answer to Audience Response Question #1
The definition of BCG unresponsive or historically refractory non-muscle invasive disease includes all of the following except:
- A. Prior BCG induction and at least one course of maintenance?
- B. T1 high grade after induction of BCG only.
- C. BCG intolerant.
- D. High grade recurrences within six months of the last BCG
- E. Positive cytology in biopsy proven CIS six months after last dose of BCG.
(Twitter Question) Reveal the Answer to Audience Response Question #2
Which of the following drugs is FDA approved for treatment of BCG unresponsive CIS? (Dr. Kamat’s lecture touched on this subject.) Hint: it was approved in 1998.
- A. BCG
- B. Valrubicin
- C. Mitomycin
- D. Interferon
- E. Gemcitabine
BCG Unresponsive Disease—A Roadmap for Drug Development and Integration of Novel Therapies
Click on slide to expand
Disclosures
This doesn’t have all of my disclosures for some reason or other, but in talking about trials in the BCG unresponsive, state we have funding through the Southwest Oncology Group trial that Peter Black and Parminder Singh are heading from Roche Genentech for Atezolizumab, that is through NCI. There’s a clinical trial that we are also involved in just as a site with FKD. VIVENTIA, and I will be just talking briefly about those.
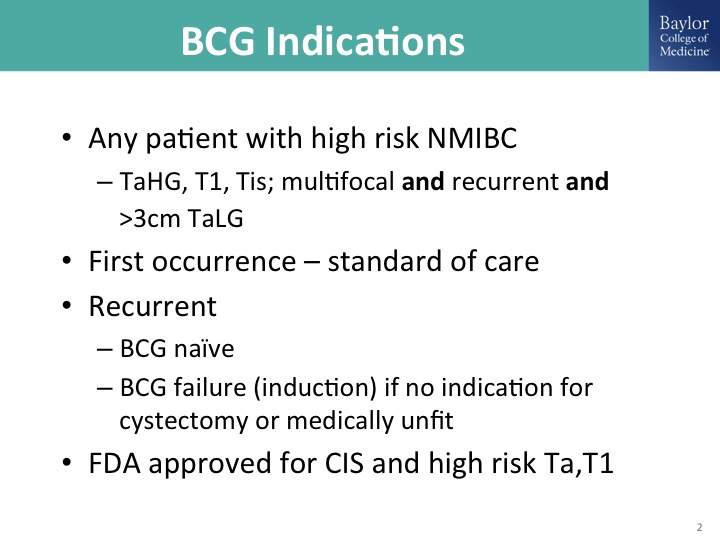
BCG Indications
So it always helps to start with indications for BCG and I have listed them here, and I think Dr. Kamat outlined them quite nicely and I too like the simple risk stratification. It’s basically indicated for anybody with high-grade disease for whom intravesical therapy is appropriate, and so that is going to be high-grade Ta, T1 after re-resection, multi-focal and recurrent, this sort of high-risk/intermediate risk disease that Ashish described. Standard of care for first occurrence. Patients who recur after an initial induction for whom BCG continues to be appropriate therapy and you can give another round of BCG. Oddly enough there has really never been a properly designed, randomized trial to address BCG in that specific state, meaning say a patient who has persistent or recurrent CIS after induction only. You can salvage a lot of those patients with additional BCG with or without interferon, and fortunately BCG has an FDA-approved indication for CIS and high-grade papillary disease.
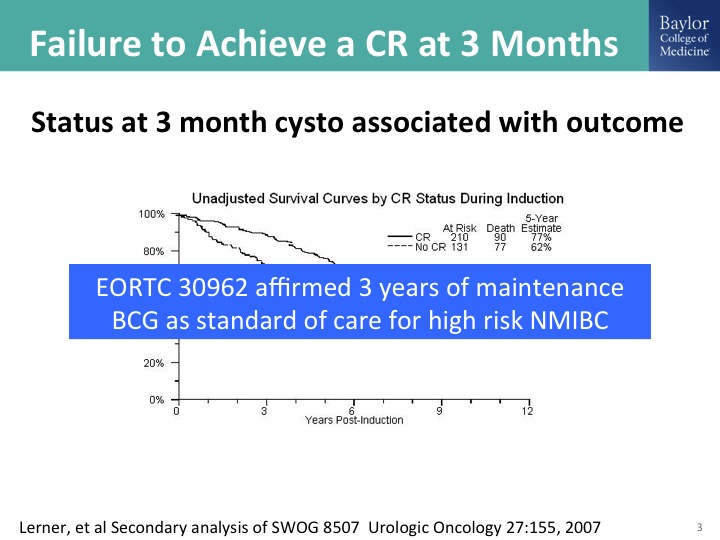
Failure to Achieve a CR at 3 months
Ashish I think also pointed this out, and this is really important to nail down as accurately as possible is the status of the bladder at the three-month mark. So that is generally six weeks after induction with BCG. This was a secondary analysis of the BCG maintenance trial that showed quite clearly, and remember the outcome here is survival. That patients with persistent disease at that three-month mark have a long-term diminution in their survival probability. The flip side of that is if you have an initial CR, and we could perhaps expand that to the six-month mark because of the sometimes delayed response to BCG. Those patients clearly have a much more favorable outcome. It seems intuitive, but it is also supported by data from this randomized clinical trial, and then there was as clinical trial done by the EORTC, which looked at, it was 2×2 study of full dose or reduced dose BCG and then the long-term maintenance at three years and short-term maintenance at one year, and it affirmed the need for three years of maintenance in patients with high-risk disease. So if you are using BCG for the patient with high-grade disease, the standard of care is induction plus three years of maintenance with the caveat that in the EORTC study roughly about a third of the patients made it out to three years. In the SWOG study 16% made it out to three years. There was no dose reduction strategy in the SWOG trial, and obviously a lot of those patients fell off because of recurrences.
Cystectomy is the standard of Care for Patients Who are Medically Fit
It is really important to understand what our guidelines say about what to do when the patient has had an adequate run at BCG, and you will see that that constitutes at least induction plus one round of maintenance, that the standard of care from our guidelines and this is not just the AUA guidelines, is a radical cystectomy. The reason for that is because the progression risk is significant enough and radical cystectomy has an extraordinarily high success rate in terms of long-term survival when you do it, and I’ll show you a slide on this while the patient still has non-muscle invasive bladder cancer. So when you have this conversation with the patient, you always outline the standard of care, but I think that all of us acknowledge that number one there’s a lot of patients for whom we have a lot of trepidation about doing that because of comorbidities and risk. Patients are not lining up outside our office door to have a cystectomy, and quite frankly there’s a lot of patients for who the treated natural history you still have a window where you may be able to cure them with a different type of therapy. That of course is the focus of this talk.
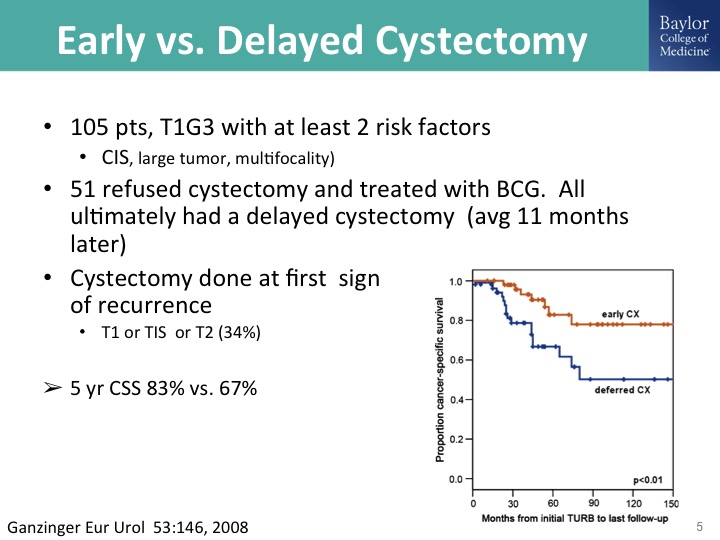
Early vs. Delayed Cystectomy
Here is the problem- it’s that we do not require histologic evidence of muscle invasive bladder cancer to justify doing a radical cystectomy, exposing the patient to that risk, and this is what the problem is. If you do a cystectomy when the patient still has muscle invasive bladder cancer, a so-called early cystectomy, I think some of us would call that an appropriately timed cystectomy as opposed to doing a cystectomy when there is muscle invasive cancer, acknowledging some of the challenges with getting accurate clinical staging, is you take a hit a big hit of about 20% decrease in their survival probability and so we have to understand as a community what the proper timing for cystectomy and be willing if not personally to do that to refer them in to centers where they do any of that instead of the not infrequent scenario that Tom Keane was sharing last night where the patient has a T1 cancer, maybe they do or don’t get resected, they don’t get BCG, and big surprise you see them when they have the muscle invasive cancer, so anyway so remember that data in your head.
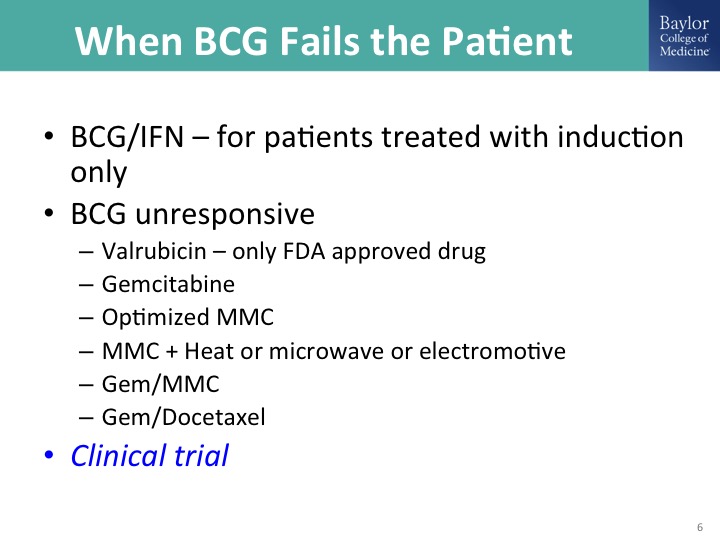
When BCG Fails the Patient
This is phraseology used by Diane Quali who is the immediate past president and co-founder of – – , she wants us to—she doesn’t want us to call this BCG failure indicating that somehow the patient has failed the treatment has failed the patient. That’s why it’s when BCG fails the patient. What can you do? So BCG failure is induction only. Just the terminology, and those patients as I have mentioned already may respond to additional induction BCG, three weeks of maintenance in the setting of CIS, and I like adding interferon. Remember that one of the ways that BCG works is inducing TH1 response mediated in part by interferon gamma, so here we are giving the interferon. We know the interferon works as a single agent, but the two drugs together it could be more potent in terms of stimulating the appropriate immune response and it does allow you to reduce the dose of BCG to help manage the potential toxicity.
So there are these—I mentioned that valrubicin is the only FDA approved drug. These other approaches, doublet therapy that Mike O’Donnell has promoted, taking a page from the medical oncologists, are all non-approved and quite frankly supported in one way or another with modest level evidence and I think today I’ll show you some data, a lot of us are rather enthusiastic about the Gen/Doce combination. So these would be—aside from valrubicin, the off-trial options that you can do now and in my talk there is the publications to guide you through that. And I’ll show you some data, and of course we’re clinical trialists, so we try to put these patients on clinical trials whenever possible.
Valrubicin
I will skip over this, because Dr. Kamat already showed you the data with valrubicin.
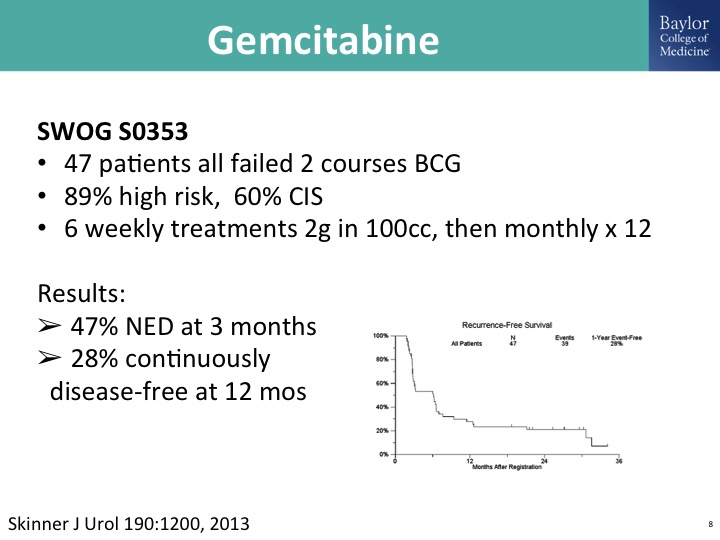
Gemcitabine
Gemcitabine has been studied in multiple trial settings, and there is probably a publication track record of over 2000 patients where this is a single agent. This is just one that was done by the Southwest Oncology Group in previously treated BCG patient population, and meets sort of this elusive FDA bar if this were going for a drug approval as an effective agent in this disease state of BCG unresponsive disease. As you know, gemcitabine is off-patent, so you can use this as an intravesical therapy either as a stand-alone drug drug or in combination with docetaxel.
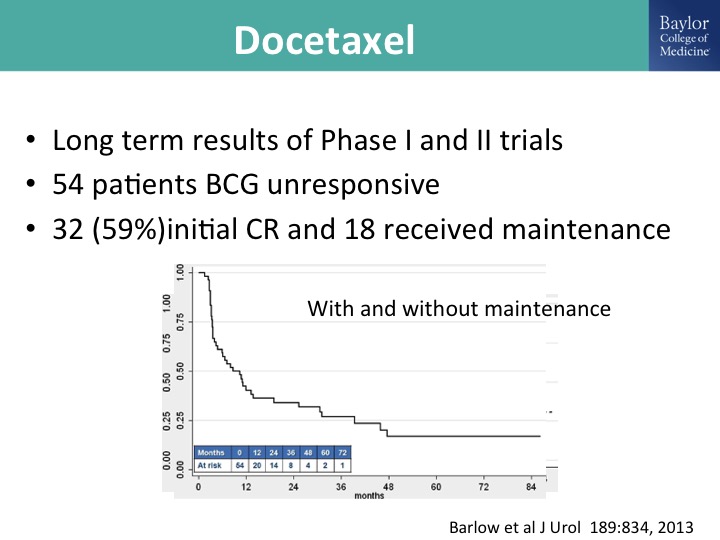
Docetaxel
Docetaxel has been studied extensively by the group at Columbia led by Jim McKearn and this is long-term results of their phase I and phase II trials, a modest number of patients and a pretty healthy initial complete response, and of those patients 18 received maintenance. This is all comers, and then this is the result with and without maintenance, and you can see that looks like an incremental gain over valrubicin, it looks pretty good with gemcitabine.
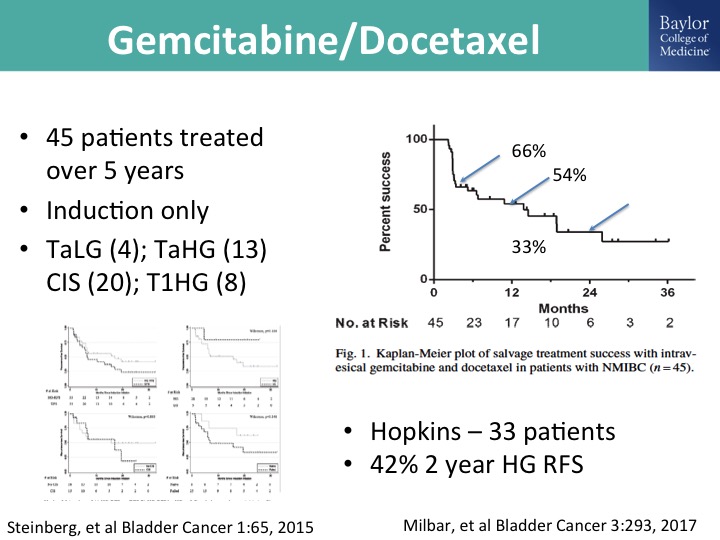
Gemcitabine/docetaxel
And Mike O’Donnell, as I mentioned, has looked at this in combination. This is his group’s report, 45 patients treated over 5 years, and you can see that at two years roughly about a third of the patients have maintained persistent, complete response, durable complete response, and then there is a nice paper that was just published form the Hopkins group, kind of a mixed bag of patients but also showing the potential efficacy and durability of this gem/doce combination, and we and many others are using this in the clinic and I think so this would be kind of currently our off-trial go to combination for patients in whom we feel like it is appropriate to not do a cystectomy straight away.
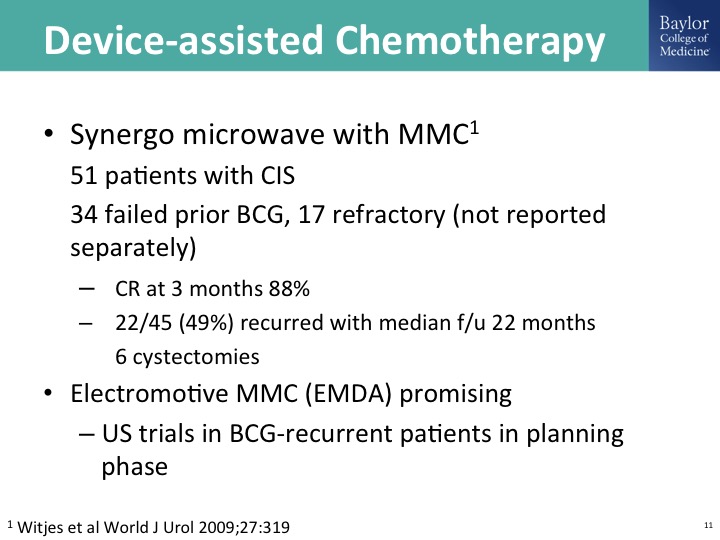
Device-assisted chemotherapy
A lot of exciting work done with a variety of ways to drive Mitomycin C into the wall of the bladder. You can do this with electrical current, so-called electromotor, this is – – work from Italy. He has done this alternating with BCG. Synergo is a device that I think was developed in Israel. It’s largely used in Europe. I think—is it used in Canada? There are clinical trials happening in the U.S. but this is a way of delivering heat, and then microwave has also been used, and so there is some pretty impressive data out there using Mitomycin C and one or more ways of device-assisted therapy. This is just one trial from Fred Witjes published in the World Journal of Urology.
Draft FDA Guidance – November 2016
Ashish alluded to this guidance document that now is aging unfortunately it was first put out for comment in November of 2016. And Jonathan Jarow would probably kill me for saying this, but I emailed him and I said what’s up. And this was what he told me, it’s still a work in progress, and I have the email to document that this was a quote from him. I think he is being fairly generous, and oh by the way he was communicating with me on the day of the government shut down, and he is a government employee so go figure. But in all fairness, Jonathan Jarow is a urologist, he’s one of us, and he has been probably one of a handful of people within the FDA who has really been our advocate, and quite frankly I don’t think we would be where we are today particularly in clinical trials in this state, disease state without Jonathan’s really guiding hand in helping educate the decision makers in the FDA to help us.
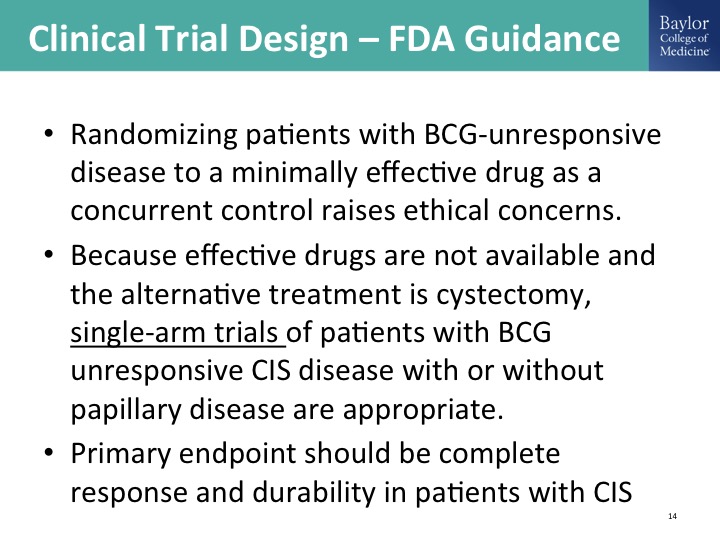
Clinical Trial Design—FDA Guidance
That guidance document sort of outlines the appropriate clinical trial design allowing for a single arm registration study, which we have seen this happen in advanced disease in IO space, immuno-oncology space. We’ll hear about it later today but this is going on in this BCG unresponsive disease state. Why is that? Because we convinced him there was no appropriate comparator. The single FDA approved drug just really isn’t good enough to be a comparator that we would be comfortable randomizing patients to that, so we have this disease state defined. We have the registration pathway designed and guess what happens? Pharma just descends on this, and in addition to pharma our own investigator initiated work, not mine personally but the community is all over this space, and this is really, really exciting.
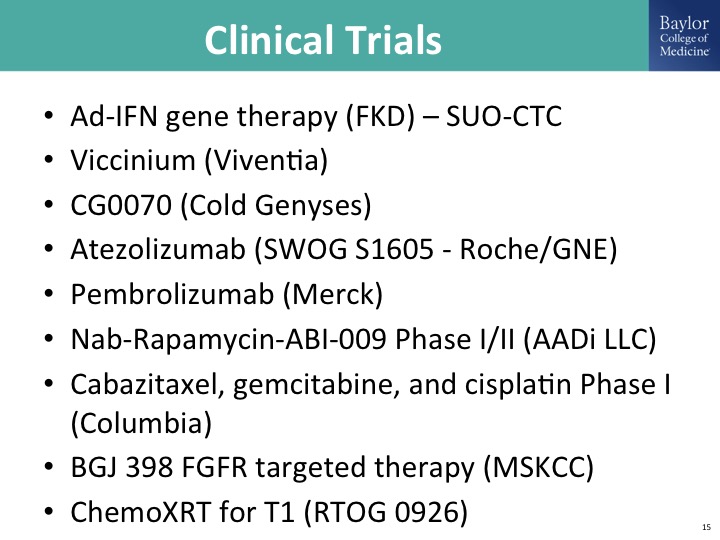
Clinical Trials
I am sure this is not a complete list of ongoing clinical trials, and there’s—I’m going to talk about a couple of them. Gene therapy, Viccinium takes advantage of EPCAM expression on tumor cells and links that to Pseudomonas or diphtheria toxin to kill the cells. I’ll talk about briefly the Cold Genyses study, which is another gene therapy trial delivering GCSF. I’ll talk about an immuno-oncology trial, there’s others going on and just a long list of very exciting trials going on in this disease state. The other kind of cool thing that we are seeing, we were a little bit concerned about where are all of these patients going to come from, and in our experience so far at least with some of the trials that a number of us are involved in is that this has definitely increased the pie of patients coming in and accessible for these trials. It took us three years to accrue 50 some patients to the gemcitabine SWOG trial, and I think the community is finishing the FKD trial in half that time and more than double the patients. So this is really exciting stuff.
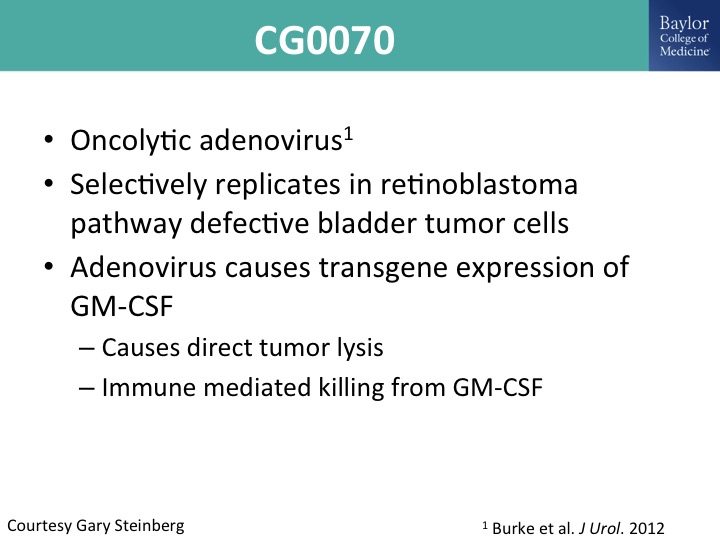
CG0070
So I mentioned this oncolytic adenovirus. This is the cell genesis. I’m not part of that trial. This was given to me by Gary Steinberg. He presented this I think at the AUA just recently as well as ASCO. This selectively replicates in retinoblastoma defective tumor dells, and it is an adenoviral mediated gene therapy delivering GM cSF, and you can see how it works there.
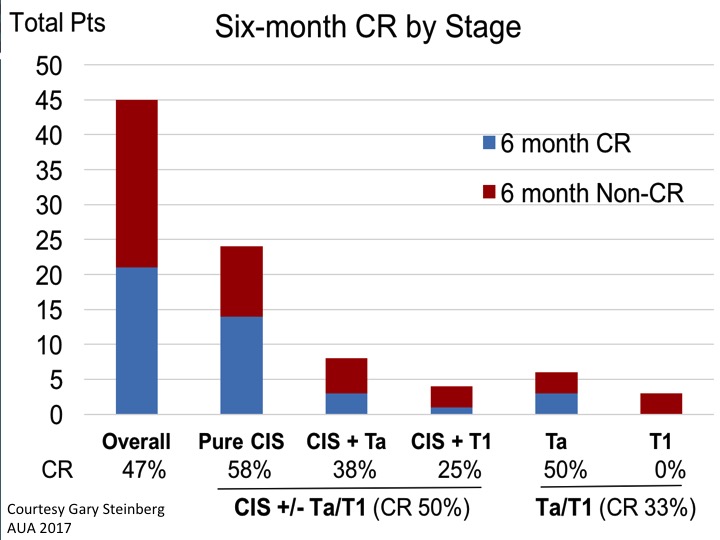
Six-month CR by Stage
This is the summary data looking at six-month complete response rate. If you look at the aggregate data of CIS with or without papillary disease and initial CR of 50%. That would certainly meet that FDA bar if it shows to be durable over time.
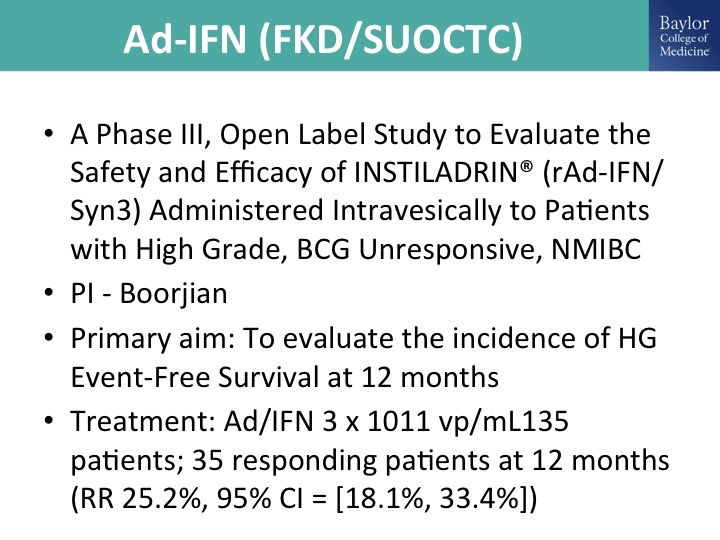
Ad-IFN (FKD/SUOCTC)
This is the ad interferon gene therapy trial, so also a replication deficient adenovirus delivering interferon. This is over a decade of work by Colin Dinny and his colleagues at the MD Anderson Cancer Center. FKD is a Finnish company that acquired the product and has been very supportive and working exclusively with the SUOCTC to do a Phase 2 trial. And the Phase 3 trial has this same standard single-arm registration pathway design, and the key thing is that we have to prove in these clinical trials efficacy in CIS, so if you think about it from a single-arm standpoint, what the FDA has been adamant about is you have to show treatment efficacy of a cancer, and the only one—otherwise if we are just treating resected Ta, T1, it’s adjuvant and there is really no way to know exactly your treatment effect without a comparator, so you have to show efficacy in CIS.
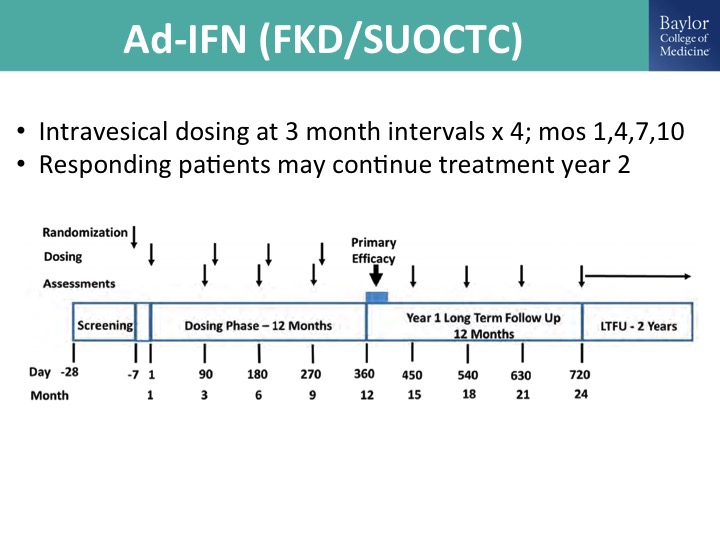
Ad-IFN (FKD/SUOCTC)
This is the trial design. I’m kind of running low on time, so let me just kind of cruise through this.
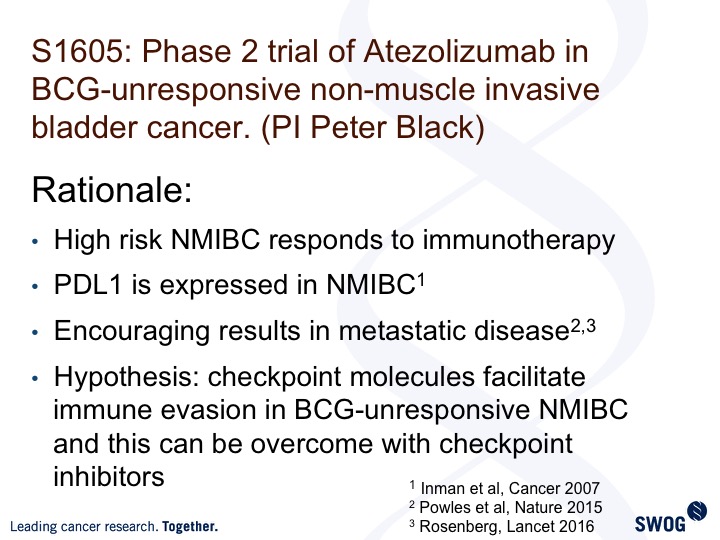
Phase 2 trial of Atezolizumab in BCG-unresponsive non-muscle invasive bladder cancer (PI Peter Black)
This is the SWOG trial, 1605, led by Peter Black and Parminder Singh. It’s looking at atezolizumab, one of the I-O agents in this same exact disease space, and it builds on the efficacy in advanced disease. It utilizes or takes advantage of the fact that PDL1 is expressed in non-muscle invasive bladder cancer, and there is the hypothesis for you.
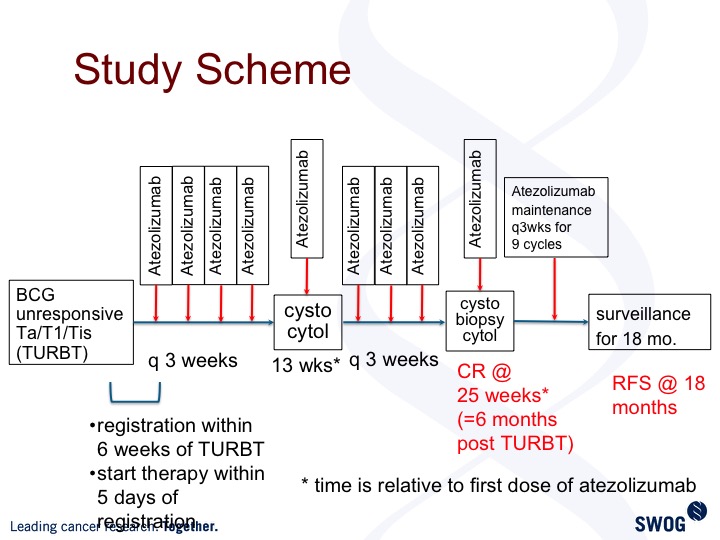
Study Scheme
This is the trial design. Patients get treated every three weeks. Standard cystoscopy evaluation at three-month intervals, and we are powered to look at complete response rate, six months post TURBT. This trial is open and accruing but still early in the trial history.
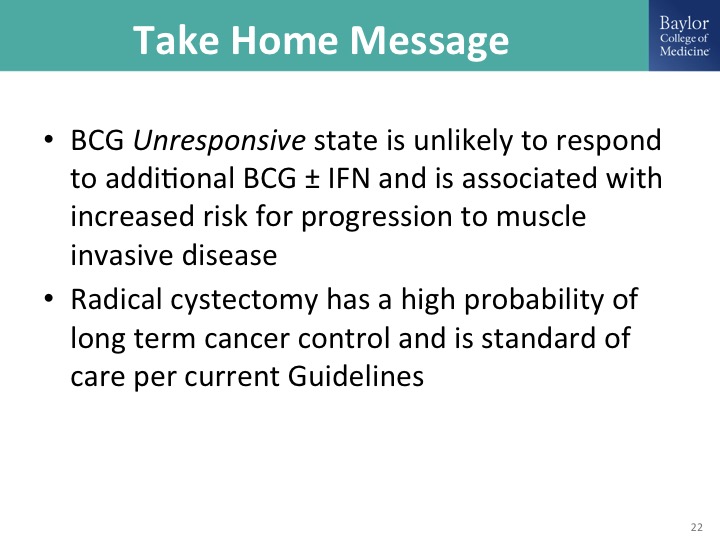
Take Home Message
Just to finish up the BCG unresponsive state defines a patient population for whom there is increased risk of progression, for whom radical cystectomy is the standard of care, but for whom BCG is no longer appropriate therapy.
ABOUT THE AUTHOR
Seth P. Lerner, MD, is a Professor of Urology and holds the Beth and Dave Swalm Chair in Urologic Oncology in the Scott Department of Urology at Baylor College of Medicine. He is also Director of Urologic Oncology and the Multidisciplinary Bladder Cancer Program and Faculty Group Practice Medical Director for the Urology Clinic at Baylor. He earned his medical degree from Baylor College of Medicine, completed a surgical internship at Virginia Mason Hospital in Seattle, and returned to Baylor for his residency training. He completed a two-year fellowship at the University of Southern California in Urologic Oncology and Reconstructive Surgery under Peter Jones and Don Skinner before returning to join the full-time Baylor faculty in 1992. His clinical practice, education, and research activities are devoted to urologic oncology, particularly lower and upper tract urothelial cancer. Dr. Lerner is an author on over 190 peer-reviewed articles, and co-editor of the comprehensive Textbook of Bladder Cancer. He is the founding Co-Editor-in-Chief of the Bladder Cancer journal. He established and directs the multi-disciplinary Bladder Cancer Research Program at Baylor, and his research interests include the use of selective estrogen receptor modulators for the treatment of bladder cancer, gene therapy, integrated genomic analysis of bladder and upper urinary tract cancers, and outcomes of radical cystectomy and pelvic lymphadenectomy. He has 25 years of experience as a clinical investigator for both NCI and industry-funded clinical trials. He is the PI of the ongoing SWOG NCI Phase III trial comparing extended vs. standard pelvic lymphadenectomy at the time of radical cystectomy. He is Chair of the Local Bladder Cancer Committee of SWOG, founding and former Co-Chair of the NCI Bladder Cancer Task Force and current Co-Chair of the NCI CTEP Genitourinary Steering Committee, and he has co-chaired the Analysis Working Group of The Cancer Genome Atlas Project for muscle-invasive bladder cancer for the past decade. He is very active in the Bladder Cancer Advocacy Network (BCAN) as a member of the Board of Directors, and is Past Chair of the Bladder Cancer Think Tank and Co-Chair of the Management Committee of the Bladder Cancer Research Network. Dr. Lerner is an active member of the prestigious American Association of Genitourinary Surgeons and is listed routinely among “America’s Top Doctors” and “Best Doctors in America.”