Dr. Michael S. Cookson, MD, presented “Enhanced Techniques of Non-Muscle Invasive Bladder Cancer Detection” at the 2nd Annual International Bladder Cancer Update on January 27, 2018 in Beaver Creek, Colorado
How to cite: Cookson, Michael. “Enhanced Techniques of Non-Muscle Invasive Bladder Cancer Detection” January 27, 2018. Accessed Jan 2025. https://dev.grandroundsinurology.com/enhanced-techniques-of-non-muscle-invasive-bladder-cancer-detection/
Summary:
Michael S. Cookson, MD, describes the components of a quality transurethral resection of bladder tumor (TURBT), enhanced detection technologies such as blue light cystoscopy and narrow band imaging (NBI), and the induction of these new technologies into the American Urological Association (AUA) and Society of Urologic Oncology (SUO) bladder cancer guidelines and common practice.
(Twitter Question) Reveal the Answer to Audience Response Question #1
In general, repeat resection is indicated in all of the following patients EXCEPT:
- A. High grade Ta
- B. High grade T1
- C. Variant histology
- D. Low grade Ta
Reveal the Answer to Audience Response Question #2
Blue light cystoscopy with Cysview, as compared to white light, has demonstrated improved detection of:
- A. TA but not T1
- B. Improved detection of TA and T1 but not CIS
- C. Reduced recurrence rates
- D. Improved overall survival.
Enhanced Techniques of Non-Muscle Invasive Bladder Cancer Detection
Click on slide to expand
Learning Objectives
So we’ll go ahead and review. Some of the objectives of this are to review the components of a quality TUR, limitations of white light cystoscopy, understand some of the technologies that are available for us to do enhanced visualization of these tumors including new data on flexible office cystoscopy, understand how these impact on the outcomes for patients including recurrence, progression, etc., and then review some of the guidelines as mentioned. So we’ll go ahead and do that.
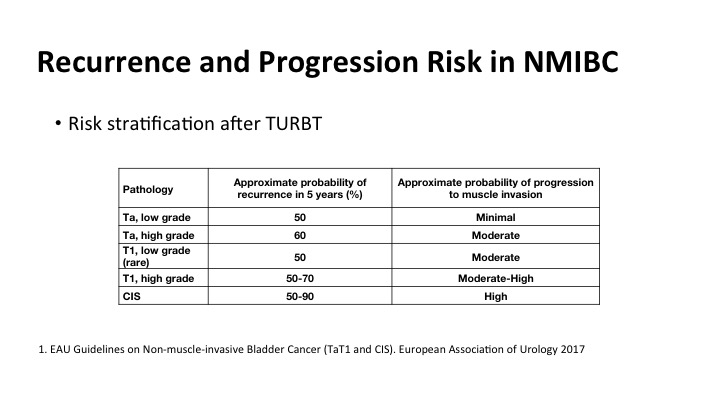
Recurrence and Progression Risk in NMIBC
We all know that as has been shown by some of the previous speakers that there’s a significant amount of risk of recurrence and risk of progression, and it is correlated highly with many clinical factors including the grade and stage of the tumor, but what is really not well documented is the risk of recurrence if tumors are not properly detected at the time of the initial TUR. And that impacts on patient outcomes.
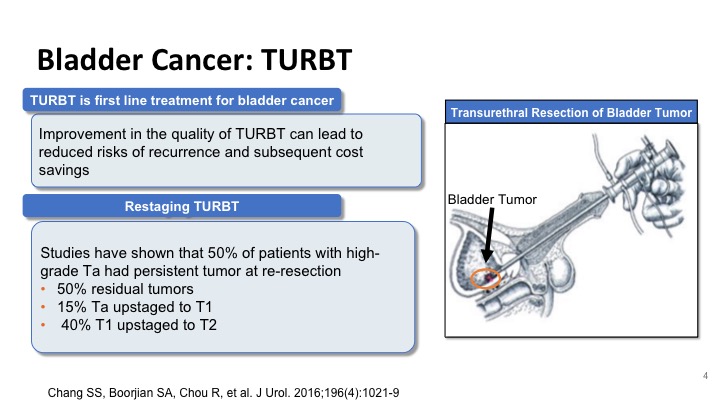
Bladder Cancer: TURBT
So improvement in the TUR can lead to a reduction in recurrences and a cost savings to the patient and the healthcare system, and studies like the one that was shown by Dr. Kamat have shown that at least half of the patients with high-grade disease at the time of repeat resection will have residual disease, and these include Ta tumors that can sometimes be up staged to T1, and T1 tumors that can be up staged to T2. And these have significant implications on how we treat these patients.
High Quality TURBT
So what are the qualities of a high quality TUR? We think first of all it’s both diagnostic and therapeutic. There is a learning curve to it, and luckily with our high definition cameras, it’s a lot different than it was in the old days, when you you were just looking through the keyhole. But there’s no real substitute for a quality TUR and complete resection. You can’t make it up with intravesical therapy.
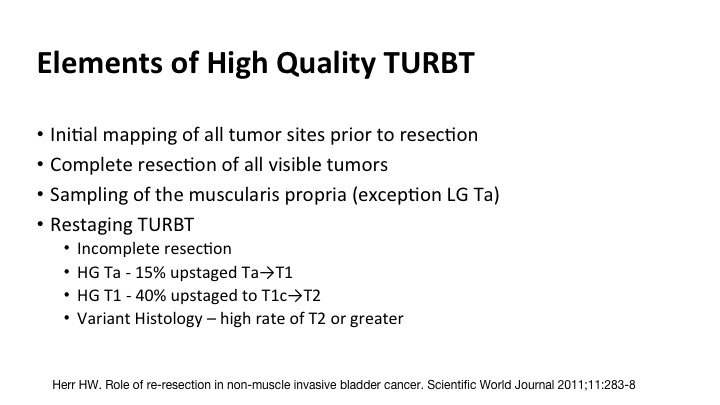
Elements of High Quality TURBT
So the components include initial mapping of all of the tumor sites, complete resection of all visible tumor, sampling of the muscle where it is appropriate, the exception might be a low-grade Ta, and then re-staging, and most of you the message has gotten out but re-stage for high grade Tas, high grade or any T1s, and certainly this new emergence of the understanding of the pitfalls of variant histology.
Elements of High Quality TURBT #2
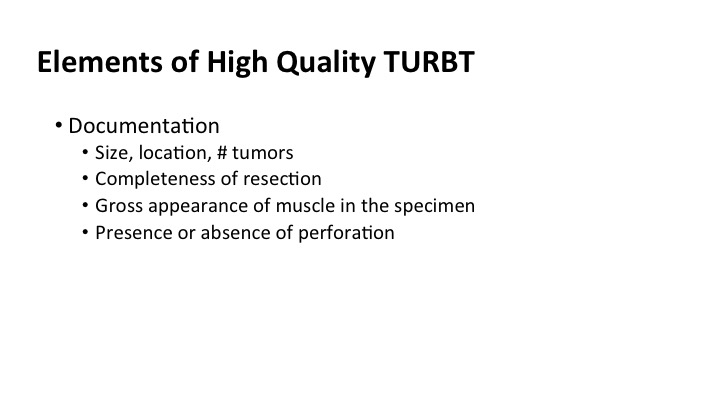
In the operative it’s amazingly sparse sometimes. I think that we could do a lot better if we had more of a templated quality note, that included size, location and number of tumors, the completeness of the resection, whether there was muscle present in the specimen that you saw because the pathologist may not report it, and the presence or absence of perforation of the bladder at the time of the surgery.
Elements of High Quality TURBT #3
So in summary, the elements of a high quality TUR include visualization, complete resection, muscle in the specimen, restaging where appropriate, and certainly documenting what you’ve done, and we’ll go back to the top now, visualization. That is really what we’re going to talk about today.
Is white light good enough?
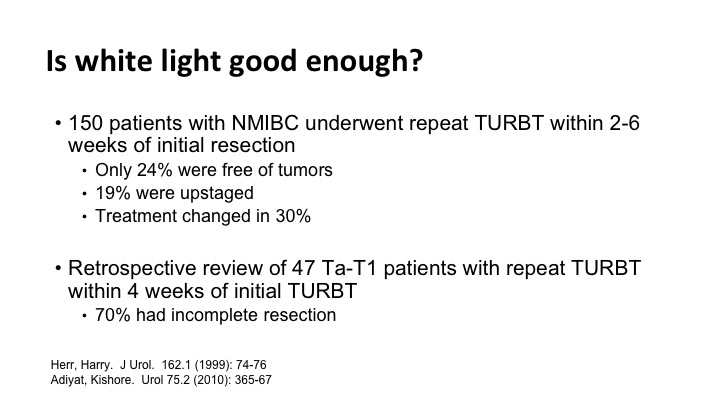
Is white light good enough, and the answer is often not. There are several studies, and there are just two of them that show that we miss tumor when you simply go back and look for six weeks later you find significant tumor. Many times the tumor that you find can change the treatment.
Enhanced Technologies to Detect Bladder Cancer
So there are several enhanced technologies out there available to you, and these include narrow band and fluorescent cystoscopy. Those are the two we’ll focus on in the limited time we have. There are others out there such as the optical coherence, and confocal laser microscopy, but those are really in development right now, and I don’t have experience with those.
Narrow Band Imaging (NBI)
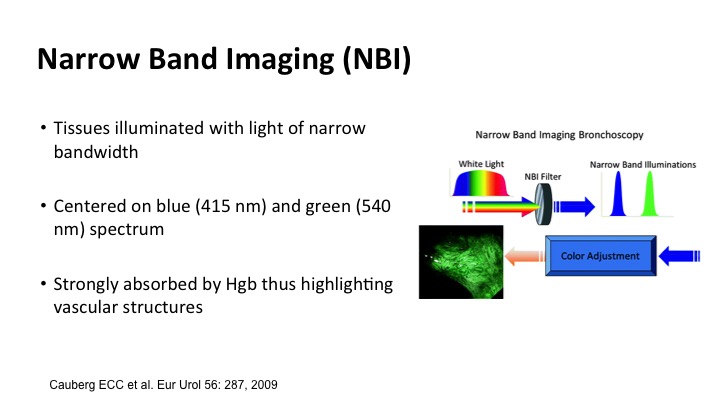
Narrow band imaging allows for taking advantage of the fact that tissues illuminate with light in a narrow bandwidth, and if you focus on blue and green the spectrum where hemoglobin is absorbed, you can highlight vascular structures, and that is what the narrow band technology does. This was a simple drawing, but it made a lot of sense. The little tumors show up as sort of brown tumors on the surface, and the deeper blood vessel capillaries are more green, so it’s kind of like being on a shallow beach.
Detection and Recurrence with NBI
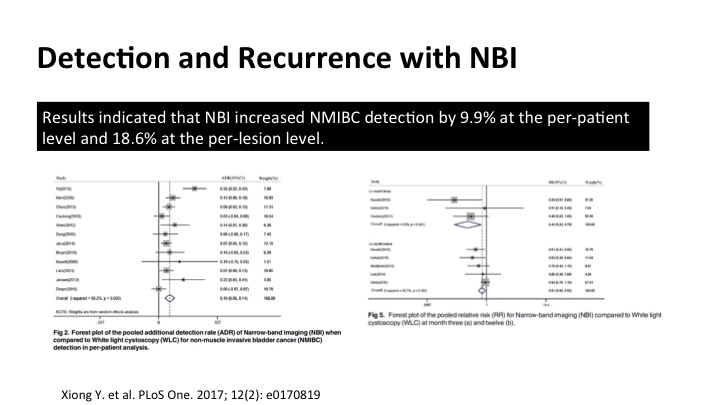
The data for narrow band hasn’t been as robust as the data for the fluorescent cystoscopy that I’ll show you in a minute, but if you cobble together a lot of the stuff into meta-analysis such as this, you can see that there is significant increased detection of tumors using narrow band, both in per patient and also additional lesions, and on these forest plots, the one to my left here shows enhanced detection definitely, and it does translate into a reduction in recurrence as shown on the far side.
Narrow Band Imaging in UTUT
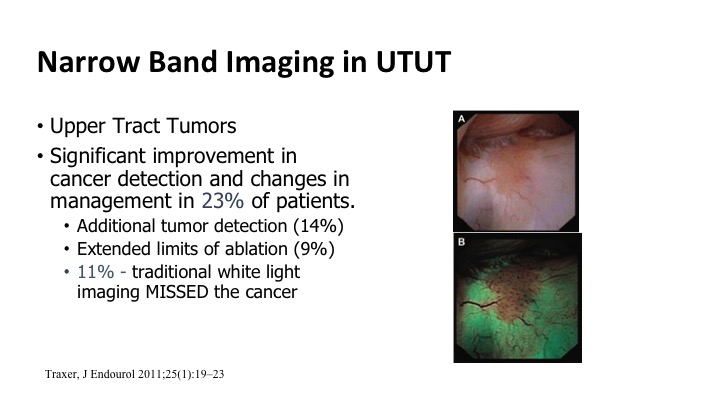
It also can be used for upper tract tumors. So the same technology can be used to go up, look in the upper tract, and this ties in with what Dr. Lerner’s talk was earlier this morning. Improvements based on some of these small series showing up to maybe 25% of patients benefit from this enhanced detection. You see more tumors, or you can maybe more quickly eradicate the borders of the tumors because you can see them better.
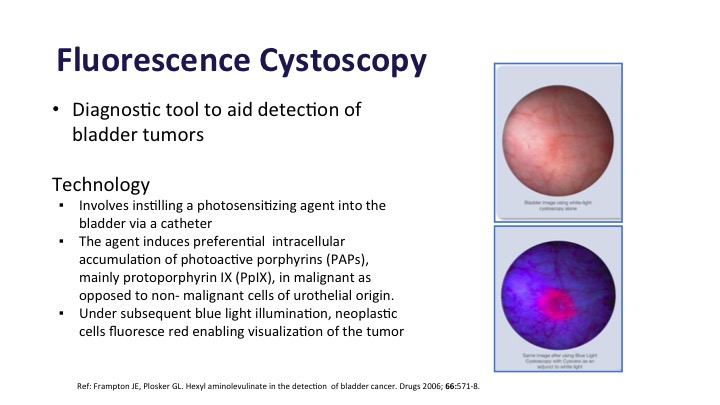
Fluorescence Cystoscopy
Fluorescence Cystoscopy is also a diagnostic tool to aid in bladder cancer detection. This takes advantage of the fact that there is photosensitizing stuff that is instilled in the bladder before patients go back for their procedure is absorbed preferentially in the tumor compared to normal bladder tissue about 10 times more. And then when you add a blue light to it, it fluoresces, and you can see the tumors.
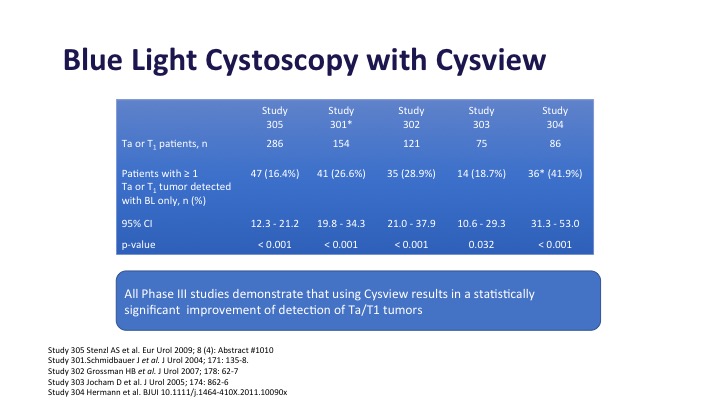
Blue Light Cystoscopy with Cysview
So unlike the Olympus, which requires just the equipment plus the Cysview kit to instill prior to the procedure, so there are sort of two parts to getting it together for the patient.
Blue Light Cystoscopy with Cysview Continued
Blue light cystoscopy in multiple studies as shown here has been shown to increase detection of both Ta and T1 tumors, anywhere from 20 to 30% depending on the series that you look at in these large phase 3 studies.
Blue Light Cystoscopy with Cysview Continued
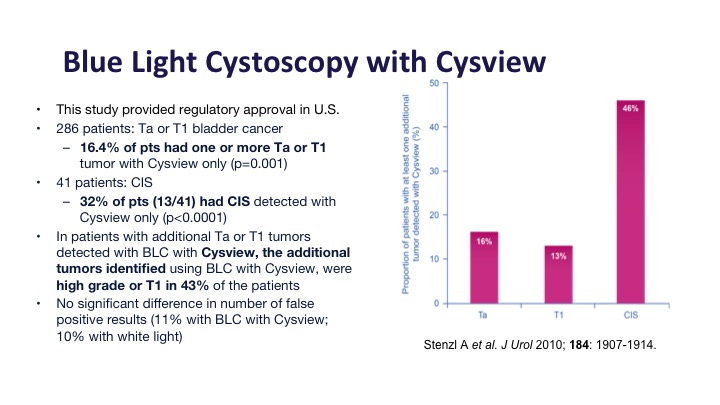
This was the registry study in the United States that got Cysview its approval, and it did show about a 16% improvement in Ta and T1 tumors and about a 30% improvement in CIS detection, and of those additional tumors that were detected about half or them, almost half of them were high-grade tumors or T1 tumors, the type that you would not want to miss.
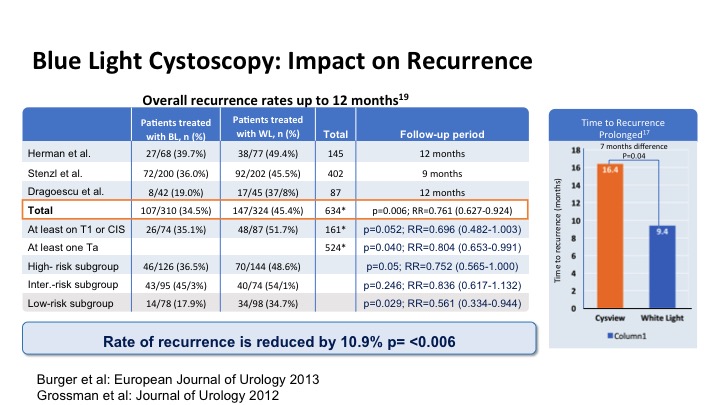
Blue Light Cystoscopy: Impact on Recurrence
This looks at the impact of that increased detection on recurrence and has been shown to significantly reduce the risk of recurrence in patients and it also has delayed time to recurrence on average by about 7 months just with the enhanced detection.
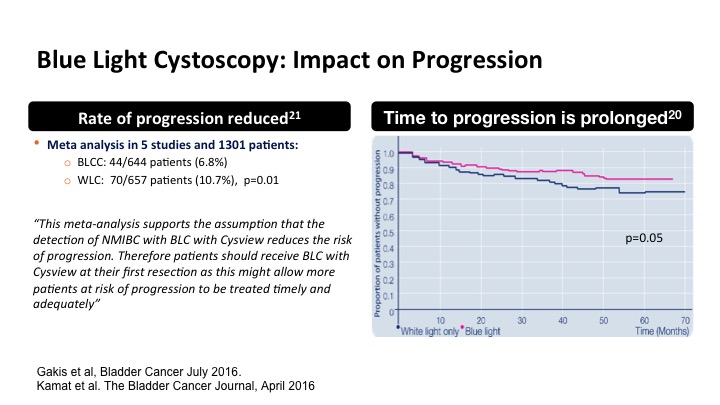
Blue Light Cystoscopy: Impact on Progression
There have been some meta analysis studies including some by Dr. Kamat and some in the audience that have suggested that this may also translate into a reduction in progression perhaps due to a more complete TUR and eradication, and also time to progression. So that is a little more borderline and kind of an interesting concept, but it makes sense if you did completely eradicate for example a T1 tumor.
AUA/SUO 2016 Guidelines: Enhanced Cystoscopy
The AUA guidelines have incorporated enhanced cystoscopy into the mix and for patients with non-invasive disease you should offer blue light cystoscopy at the time of TUR if available to increase both detection and decrease recurrence, and the level of evidence is a grade B. In patients with narrow band, you can also use it to do the same, but the strength of the evidence at the time of the guidelines wasn’t quite as strong, but nevertheless it is there.
AUA 2017 Late Breaking Abstract
This was almost published. I think it’s published online but not quite in print, but it’s coming soon to the Journal of Urology, but this was a trial looking at currently we don’t have blue light available in the clinic, so this was the blue light safety and efficacy trial using fluorescent technology in the clinic in a multi-center 17-site.
Methods
Many of you who are here, probably participated in this as we did. So they would undergo—these are patients who had a previous tumor, so this was their first cystoscopy coming back after non-invasive tumors, and they would undergo surveillance cystoscopy with either white light or blue light, it was randomized, and then if you found something they would go to the OR and do a confirmatory with the blue light.
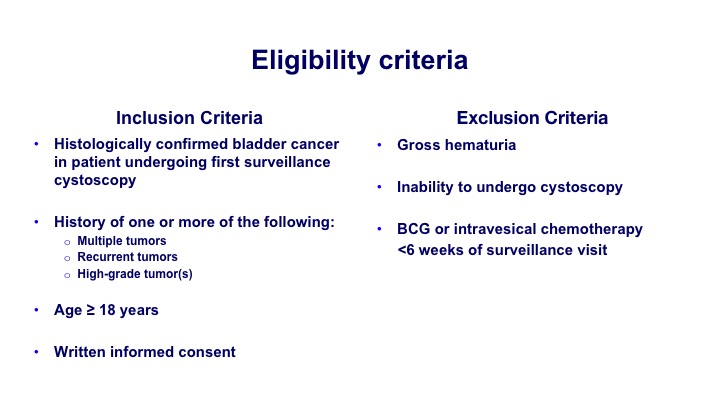
Eligibility Criteria
So again, histologically confirmed most of the patients were at risk for some degree of recurrence. So they had multiple tumors, recurrent tumors or high-grade tumors.
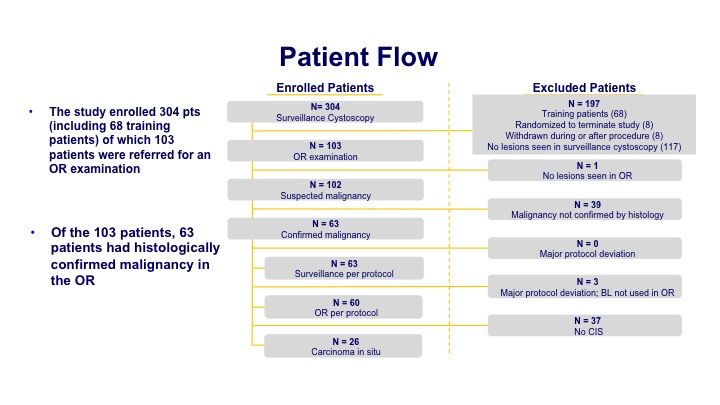
Patient Flow
The enrollment was about 300 patients of which about 100 had a significant finding at the time of the office procedure. Then that led to 63 patients when they underwent their biopsy that had histologically confirmed disease.
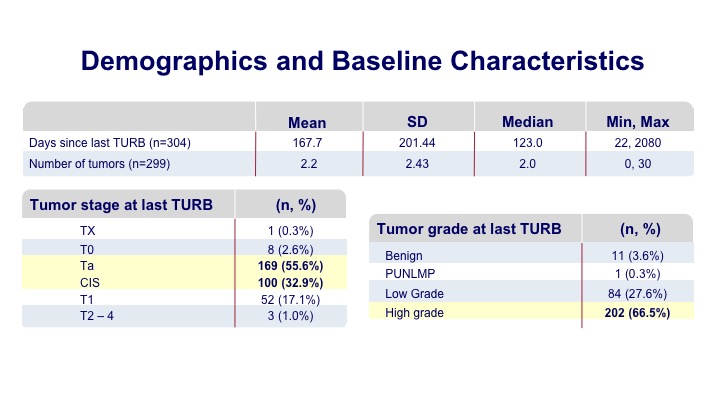
Demographics and Baseline Characteristics
Most of these patients were undergoing a surveillance within six months of their initial tumor diagnosis, and many of them had multifocal tumors prior to the discovery. Most of them were the type of patients that you would see in your office such as Ta tumors and some CIS.
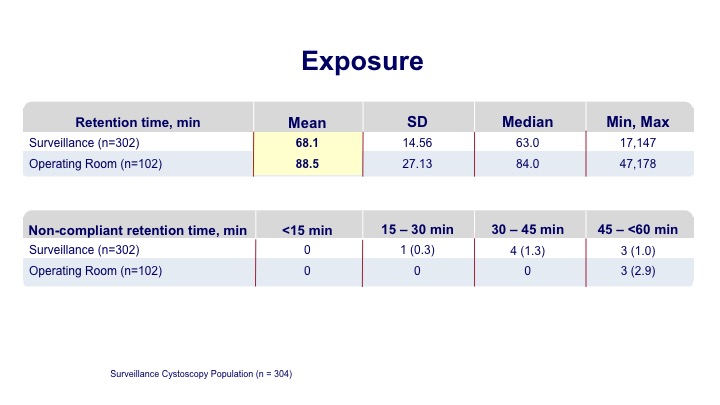
Exposure
The exposure time on average was a little over an hour for the office-based procedure, and a little longer for those going to the operating room.
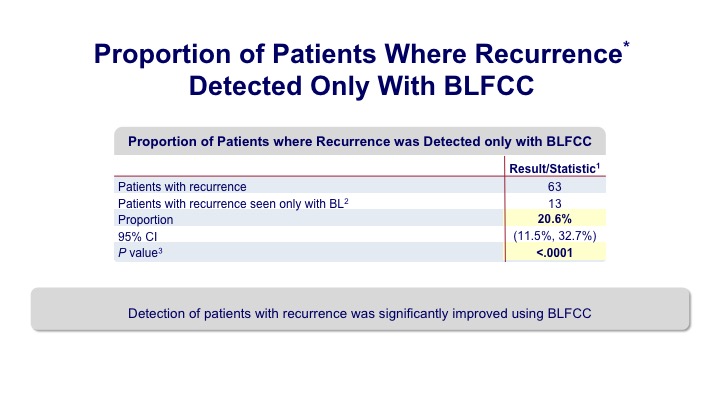
Proportion of Patients Where Recurrence* Detected Only with BLFCC
And what they found was that there was a significant proportion of increased detection in patients who underwent the blue light evaluation.
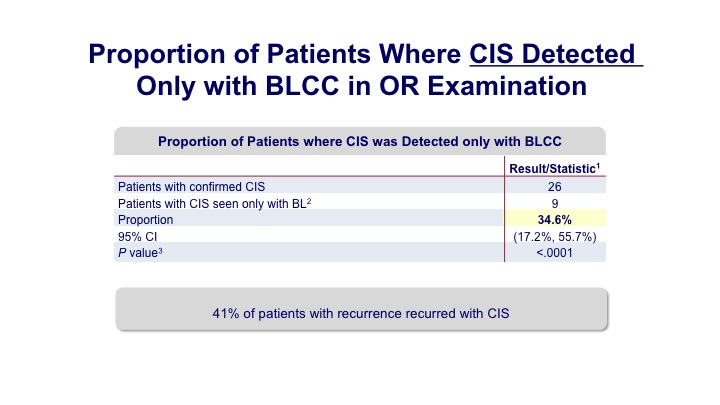
Proportion of Patients Where CIS Detected Only with BLCC in OR Examination
Not only was there a 20% enhanced detection of papillary tumors, but about a 30% detection of carcinoma in situ.
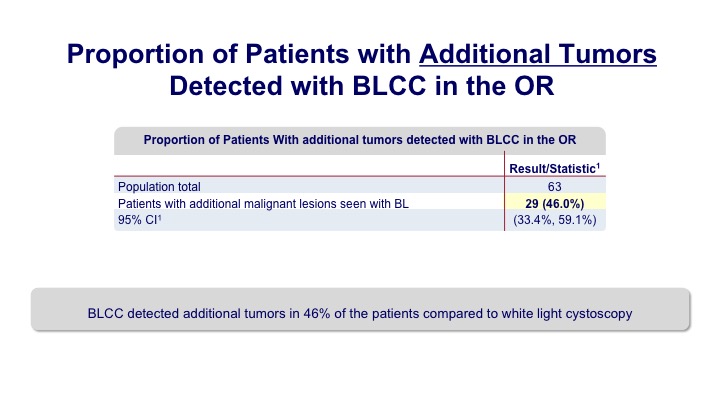
Proportion of Patients with Additional Tumors Detected with BLCC in the OR
Overall we saw that additional tumors were seen in up to about 40% of the patients. So it’s a pretty significant bump.
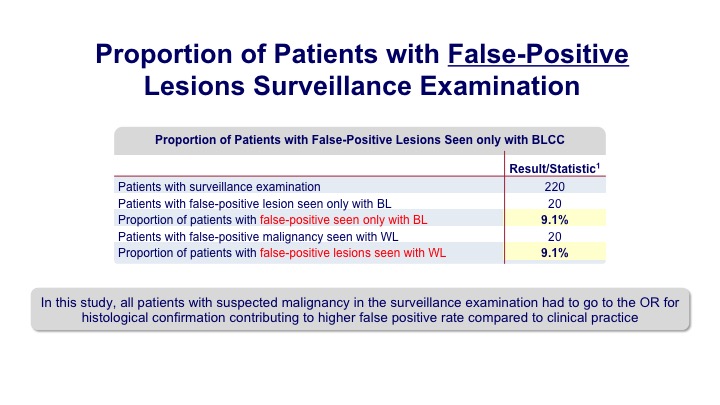
Proportion of Patients with False-Positive Lesions Surveillance Examination
There were some false indications both with white light and blue light, similar in each group.
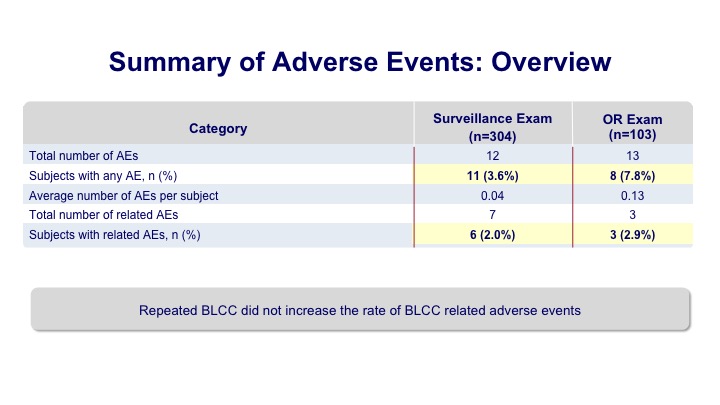
Summary of Adverse Events: Overview
And overall there were a few adverse events meaning that their repetitive use of this did not seem to translate into any problems because previous to this Cysview had sort of a single use approval.
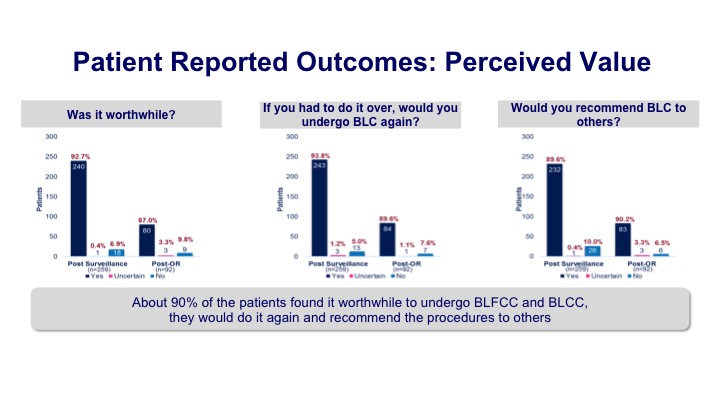
Patient Reported Outcomes: Perceived Value
These are some of the patient-reported outcomes in terms of what it was like for the patients because it does require a catheterization prior to the Cysto, installation of the medicine, and then allowing for the appropriate amount of time, usually about an hour, but patients felt like it was worthwhile. If they had to do it again, they would, and they would recommend it to others. So at least from a patient perception, it was certainly well received.
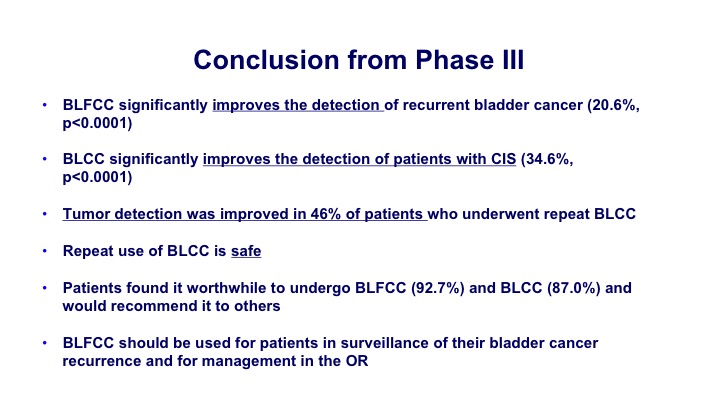
Conclusion from Phase III
The conclusion from this new Phase III study was that blue light significantly improves the detection of recurrent cancers, it improved the detection of CIS, and tumor detection was improved in 46% of the patients. It was safe and it can be used—so a couple of goals. One was just to look at the office-based detection but secondarily they wanted to look at the ability to detect it in patients who had BCG in the past. They wanted to look at the repeated use. They wanted to look at its ability to detect CIS, and for all of those primary and secondary endpoints, they were able to show benefit so they felt like this was overall a successful study.
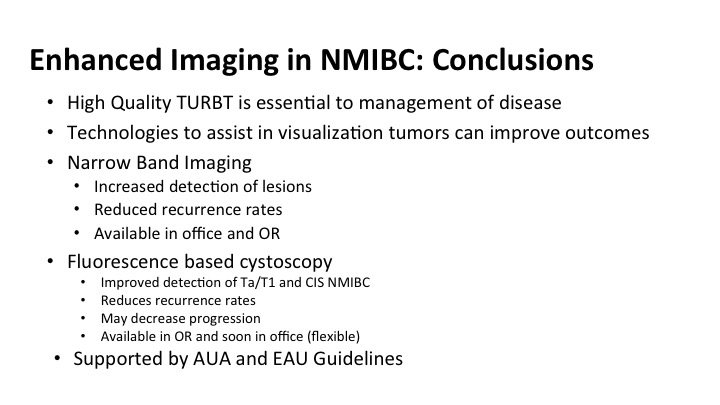
Enhanced Imaging in NMIBC: Conclusions
So in conclusion for some of the new emerging stuff with enhanced technology and enhanced imaging, I want to remind you that a high-quality TUR cannot be overestimated, and it is essential to the management. These technologies are available to you or should be soon, and are really good tools to help you do a better job of what you already fell like you do pretty well, both narrow band and fluorescent cystoscopy increased the detection of these lesions. That translates into reduced recurrence rates. Currently the narrow band is office-based and in the OR, and soon, I think not only with the fluorescence be available to you, it is available to most of you who have it in your hospitals, but will it be available as an office-based procedure as well.
These technologies given the level of evidence that is behind them are now supported by guidelines and certainly should be incorporated into the management of patients in your practice with non-invasive bladder cancer.
ABOUT THE AUTHOR
Michael S. Cookson, MD, MMHC, is Professor and Chairman of the Department of Urology and holds the Donald D. Albers Endowed Chair in Urology at the University of Oklahoma Health Sciences Center in Oklahoma City. He has authored some 240 peer-reviewed journal publications as well as more than 30 chapters of various textbooks, and he is nationally recognized for his outstanding contributions to urologic oncology. Dr. Cookson completed his Urology Residency at the University of Texas, San Antonio, and completed his Urologic Oncology Fellowship at Memorial Sloan-Kettering Cancer Center in New York. From 1998 to 2013, he served as the Vice Chairman of Urologic Surgery and Director of the Urologic Oncology Fellowship Program at Vanderbilt University. Dr. Cookson has devoted much of his academic career to the management of patients with urologic cancers, with a strong emphasis on clinical guidelines, education, and evidenced-based medicine. He was a member of the AUA/ABU Examination Committee for 10 years, serving as Oncology Consultant and Pathology Editor. He also serves on the ABU Oral Examination Committee. He is a Co-Founder of the Oncology Knowledge Assessment Test (OKAT), an SUO-mandated examination. He also served as Chair for the OKAT for 5 years. In 2011, he received the President’s Distinguished Service Award from the SUO for educational contributions. He received the 2018 AUA Presidential Citation for Outstanding Service for his role in the development of the OKAT and as Chair of the Castration-Resistant Prostate Cancer Guidelines Committee at the AUA 2018 Annual Meeting. Dr. Cookson has previously served as a member of the AUA Guidelines on Localized Prostate Cancer Committee. Dr. Cookson is currently serving out the 2019-2020 term as the SUO President.