Raoul S. Concepcion, MD, presented “Immunotherapy 101 for the Urologist” at the International Prostate Cancer Update on January 27, 2018 in Beaver Creek, Colorado
How to cite: Concepcion, Raoul S. “Immunotherapy 101 for the Urologist” January 27, 2018. Accessed [date today]. https://dev.grandroundsinurology.com/Immunotherapy-101-for-the-Urologist/
Summary:
Raoul S. Concepcion, MD, summarizes the mechanism of action behind the immune response to cancer. He also provides an update on the current and emerging immunotherapies for cancer treatment, including vaccines, checkpoint inhibitors, CAR T-cell therapies, viral vectors, and adoptive cell therapy.
Reveal the Answer to Audience Response Question #1
True or false- Coley’s toxin is one of the first examples of an autologous immunotherapy.
- A. True
- B. False
Reveal the Answer to Audience Response Question #2
Following localized radiation therapy of a solid tumor with metastatic sites, there can be resolution/shrinkage of these sites away from the primary. This phenomena is known as:
- A. Abscopal effect
- B. The Crawford Phenomena
- C. Stauffer’s syndrome
- D. Pringle maneuver
(Twitter Question) Reveal the Answer to Audience Response Question #3
The following are all cells critical to the innate immune system except:
- A. Dendritic cells
- B. Macrophages
- C. NK cells
- D. CAR T-cells
Immunotherapy 101 for the Urologist – Transcript
Click on slide to expand
Immunotherapy 101: A Guide for Urologists
Yeah, this is pretty easy, so I’m going to take a little bit of a different pathway here. We’re going to talk—this is going to be more of a didactic lecture. What I thought I would do is level set for the audience where we stand with some basics as it relates to immunology and immunotherapy, which as we all know, is really going to be sort of this burgeoning explosion of new therapy. I’ve entitled this lecture Immunotherapy 101: a Guide for the Urologist.
Objectives
And so the objectives of this talk is going to be to explain and define the mechanism of action behind the immune response to cancer, and again we’re going to talk really some basics about what the immune system does and better define the types of immunotherapy current and emerging used for cancer treatment.
Immunotherapy/Hormonal Therapy
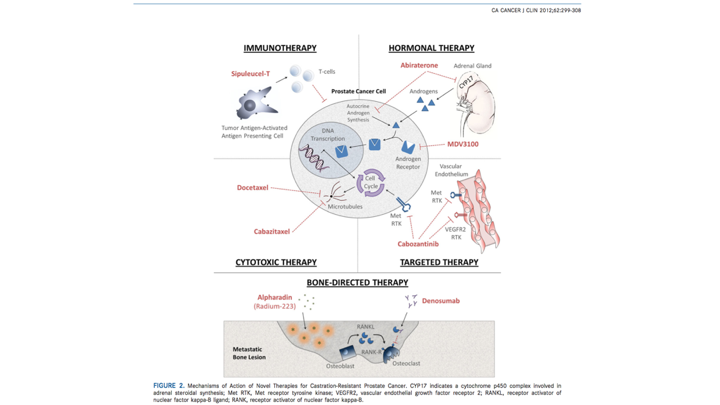
This slide really was just data that’s 2012, but basically this is really related to prostate cancer tumor cells, and so we know the canonical mechanism as was described earlier. We know it’s all driven by the androgen receptor, which involves binding of the androgen ligand to the receptor result—then you get nuclear translocation of the receptor ligand complex. DNA binding transcription and translation, so when we look at the current therapies that are available, we know that historically speaking, as we all know hormonal therapy has been sort of the foundation, and now with some of these secondary hormonal agents, including abiraterone, this was the original MDV3100, which we now know is enzalutamide, and then we have targeted therapies, cytotoxic therapies, which were your traditional chemotherapeutic agents. Then we also know because of the potential for prostate cancer to metastasize, we have bone targeted therapy, which involves not only treatment, but as well as attempts since we know that androgen deprivation therapy does result in bone mineral density loss, we also get some bone targeted agents that are available to not only prevent SRE, but also increase bone mineral density. But clearly what we know now, and again this has really been over the past few years has exploded.
Breakthrough of the Year 2013
And the one as this slide shows that was initially approved was sipuleucel T, which we know is an autologous immunotherapy, but since 2013, and again this continues to almost change on a monthly or weekly basis. This is all about immunotherapy. What I thought I would do is sort of level set for everybody sort of the basics of the immune response.
William Coley
So this is William Coley. So, Coley was a surgeon in New York. He was a general surgeon. He actually had a very specialized niche. I don’t know of anybody that does this now, but he only really took care of sarcomas, and what he observed in the late 1800s, was that there was a patient who had a metastatic sarcoma site to his neck that developed a severe skin infection and it actually had spontaneous resolution of the tumor.
So, Coley postulated that possibly this was related to this inflammatory response. So what Coley did, and really this is kind of one of the true first clinical scientists, what he did is he basically injected tumor sites, most of them were inoperable sarcomas that were metastatic. He injected attenuated a mixture of strep pyogenes and another bacteria, which we now know is soracea. He basically attenuated them. He heat killed them, and he injected these directly into the tumor sites to set up this basically immune response, and so actually this was sort of the—sort of one of the first uses of immunotherapy, but it was a direct injection of hopes of creating this immune response.
The Long Pursued Goal of Engaging an Effective Antitumor Immune Response Has Recently Come Within Reach
So many of you have probably seen variations of this timeline, so Coley did his first work in the late 1800s. Then there was sort of this lack of anything, but then back in the 60s, we started using BCG. Rosenberg then started doing a lot of work at NIH with Interferon IL2, but as you can see here, as we all know the first cellular immunotherapy approved for prostate cancer was sipuleucel T in April of 2010, and since then we have had a number of different immunotherapies approved, and again this number continues to increase, and I predict will basically continue to go on for the next couple of years.
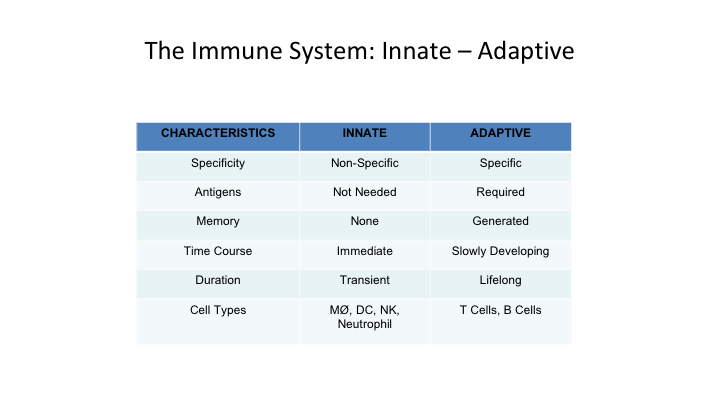
The Immune System Has: Innate – Adaptive
So when we talk about the immune system, you really have to talk about two basic concepts. You talk about an innate immune system, and the adaptive immune system. So the innate system is non-specific, you don’t need antigen presentation, it doesn’t have memory, the time frame is immediate course of action. The duration is transient, and the cell types really are macrophages, macrophages, dendritic cells, natural killer cells and neutrophils.
The adaptive system, which is really again I think more important, it is very specific. It does require antigen stimulation. It does generate memory. It’s slowly developing, and you have a lifelong duration, and its main cells really are T cells and B cells.
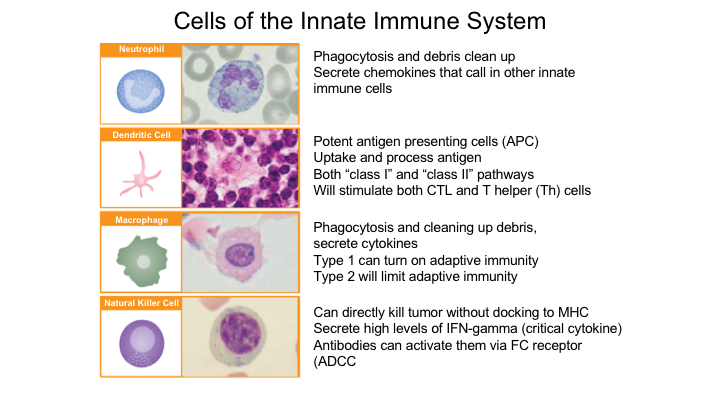
Cells of the Innate Immune System
So again, the cells of the innate system, you have the neutrophils, which are basically result in phagocytosis and debris clean-up. It secretes cytokines that call in other innate immune cells. You have these dendritic cells, which are very important to understand, especially with the current immunotherapy that is approved of sipuleucel T, and these are the antigen presenting cells. You have macrophages, and you also have these natural killer cells.
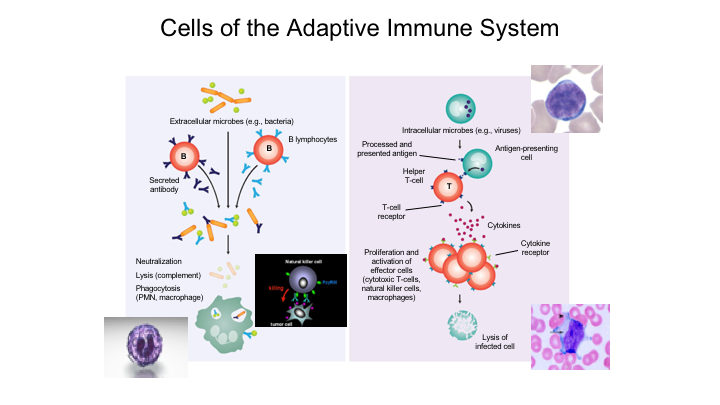
Cells of the Adaptive Immune System
When you look at the adaptive immune system, this is really based upon two types you have basically your B lymphocytes, which are responsible for specific antibody production that are generated against extracellular epitopes or microbes with specific antigen. This is again when we took immunology back in college, back in medical school, this was sort of that lock and key effect, so it results in specific antibody production, you get neutralization, lysis and phagocytosis.
The T-cell response really involves these antigen-presenting cells, uptake of an antigen, presentation and then activation of these really specific T-cells that again will have long-term memory.
Initiation of Immune Response: Key Cells
So the initiation of this is really and this is courtesy of our folks from Dendreon, is that you have these antigen-presenting cells. They take up the antigen. You get presentation of these antigen fragments for sipuleucel T, it’s actually against the antigen that is used to stimulate this activation is prostatic acid phosphatase. This actually happens in lymphoid organs. Then you have these activated T-cells, then you have these effector cells that basically result in cell death. These effector cells again are targeted. You get up regulation, and again this is antigen-specific.
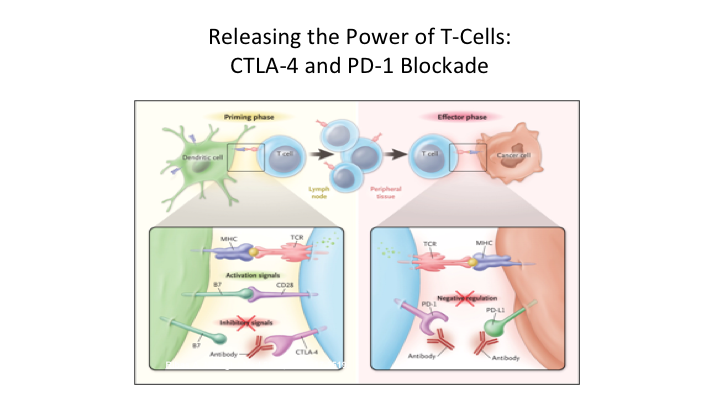
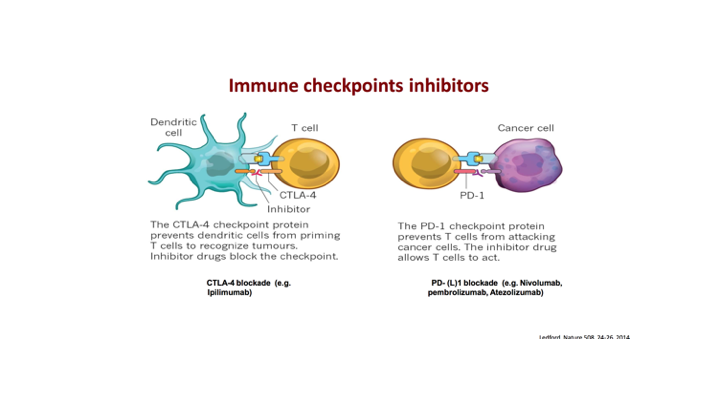
Releasing the Power of T-Cells: CTLA-4 and PD-1 Blockade
So, when you look at this at the receptor level, and this is probably the most important slide, because you hear these terms all of the time, you hear CTLA4 PD-1 Blockade. So again, what you have here, the key cell here is the T-cell. So as we showed, this priming phase is really a two-step process.
So what you have in these dendritic cells that have taken up the antigen, they present through a major histocompatibility receptor. They basically have this presentation of the specific antigen, which binds to the T cell receptor. Then you also have these other basic proteins, ignore this for a second, but you have these other basic proteins that when they also bind to certain receptors you then get activation of these T cells that become very specific. So this is sort of the priming of these T cells, and then on the effector side what tends to happen is that again you have an antigen that’s been targeted. You get binding of the activated receptor to the antigen on the cancer cell. But because of this binding over here to CD20A receptor, you get this whole up regulation. But what happens is that we have these natural proteins, these checkpoint inhibitors, and so what happens is that on the dendritic cell these checkpoint proteins when it binds to CTLA4 what that results in is basically an inhibition of the T cells. On the effector side you then have PD1 and PDL1 so if you get binding of PDL1 to PD1 then you get down regulation and it affects the—it basically knocks out the effector phase. So this is really a very important concept, and it is important to understand where this actually takes place.
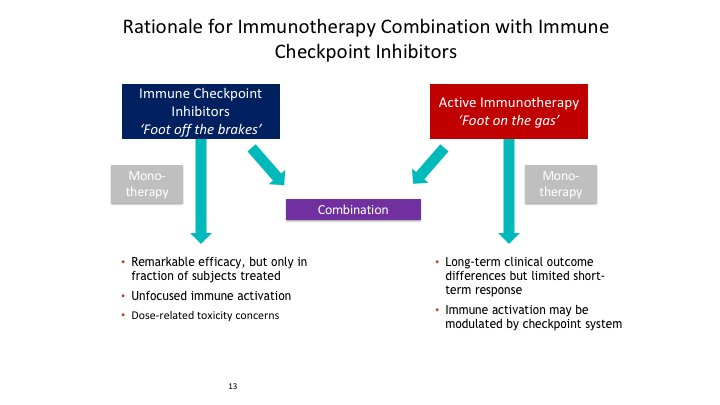
Rationale for Immunotherapy Combination with Immune Checkpoint Inhibitors
So again this rationale for immunotherapy combination with immune checkpoint inhibitors, is that you have the checkpoint inhibitors, these anti-PD1, PDL1, CTLA4 inhibitors, which really result if you block them if you block this interaction, you then release the effect and you get up regulation of all of these effector T cells. If you block PD1 or PDL1, you then block this interaction and what you get is what normally results in a negative regulatory effect.
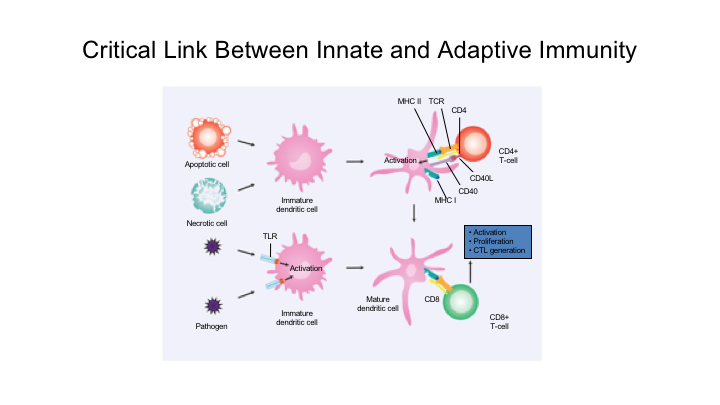
Critical Link Between Innate and Adaptive Immunity
So again, this is sort of these critical links between the innate and adaptive immunity. This is probably one of the most—this is a great little video that I thought I would just show. This really talks about this PD1, PDL1 interaction.
[Video]
I thought that was one of the greatest little videos that I’ve ever seen.
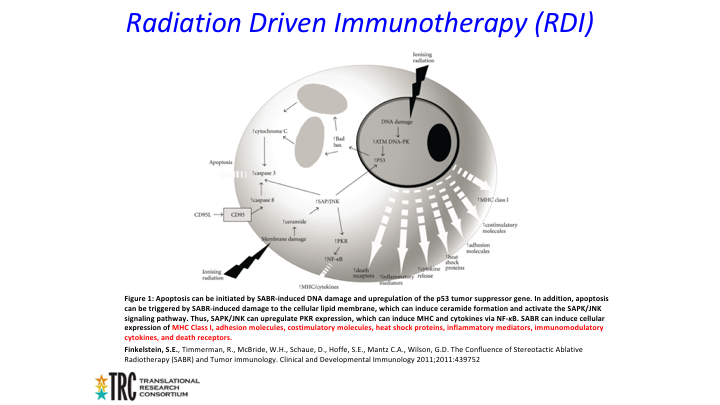
Radiation Driven Immunotherapy (RDI)
So again, these cancer cells to put it simply when they express PDL1 that binds to this receptor, that is sort of their cloaking mechanism, if you will, at the effector side, so binding of PDL1 or binding of the receptor basically takes that out and allows the T cell immunity to sort of take place.
So another area of immunotherapy is what we call radiation driven immunotherapy. We believe that basically ionizing radiation results in sort of this double stranded DNA breaks that are more difficult for the body to repair.
Radiation Driven Immunotherapy (RDI)
That sounds very simple, but there is probably a lot more going on here so again we get this ionizing radiation. We think it results in these sort of double-stranded DNA breaks, which are more difficult to repair than single-stranded breaks, but probably at the same time you get this release of all of these other molecules cytokines, receptors, adhesion molecules that result in this massive stimulation of the immune system.
Radiation Driven Immunotherapy (RDI)
So again, the most dramatic clinical effect, outcome of this, when distant tumor masses regress is sort of known as the abscopal effect, and I think Dr. Koo may be talking a little bit about this in terms of the Radium-223, which is obviously a systemic alpha emitting particle, and how it could be used essentially with other combination therapies.
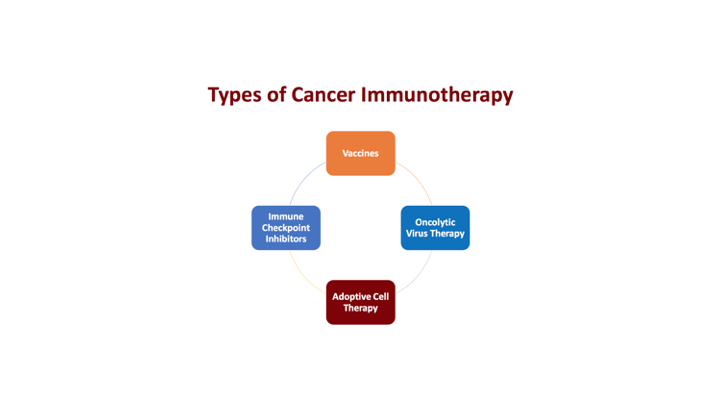
Types of Cancer Immunotherapy
When we think about cancer immunotherapy and again this is sort of a generalized way that I think people ought to be looking at this, so we have vaccines, so we have checkpoint inhibitors, there was some attempt at oncolytic viral therapies, using viral vectors, and then something new, which we’ll touch base briefly, which is becoming more and more important, is the adoptive cell therapy.
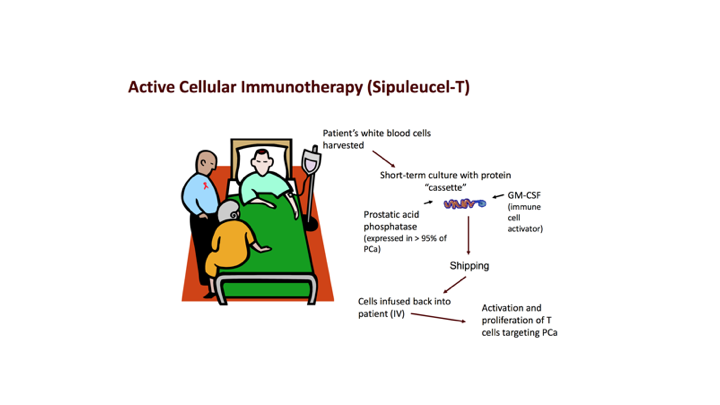
Active Cellular Immunotherapy (Sipuleucel T)
So we know that active cellular immunotherapy though again the one that is approved, and the only one that is approved for prostate cancer is sipuleucel T which was approved in April of 2010, I’m sure many of you have had personal experience, know about sipuleucel T, but really what it involves, it involves apheresis, where you pull out these dendritic cells, these antigen-presenting cells, you incubate them with a fused molecule GMCSF and basically you target prostatic acid phosphatase. You get up regulation. This process takes place ex vivo. It’s shipped back to the provider’s office, and then it is infused. As all of you know, sipuleucel T is approved and does have a survival benefit with recommendations by many of the guidelines that David talked about.
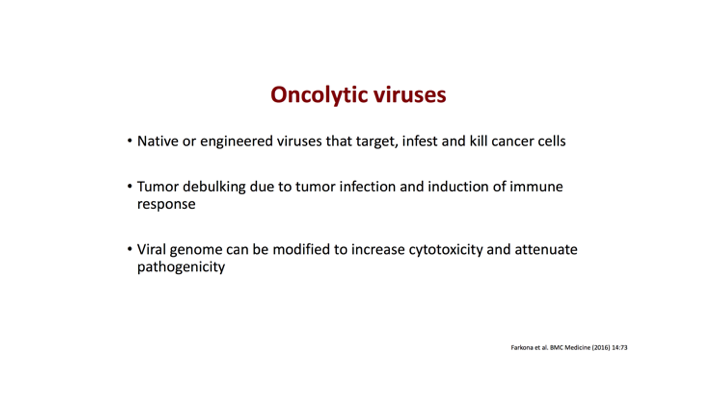
Oncolytic viruses
Again, there were other attempts to use viral vectors that have been attenuated. We know that native or engineered viruses that target, infest and kill cancer cells and unfortunately the one oncolytic viral therapy basically had a negative read out last year.
Immune checkpoint inhibitors
We’ve talked a little bit about these checkpoint inhibitors and again I think what is important to know is that the CTLA4s really target right here in terms of the priming effect, and then as we talked about in the video, the anti-PD1s and PDL1s really are at the effector side.
CAR T-Cell
Now, this new area, which again is getting a lot of press, and there’s a lot of companies that are involved in CAR T-cell therapy. There’s Juno, there’s Gilead, Kite, Novartis, is this concept of chimeric antigen receptors.
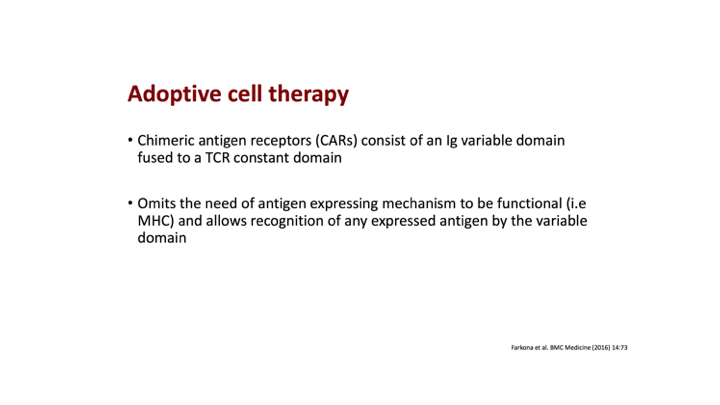
Adoptive cell therapy
Really what this is is the incorporation of an immunoglobulin variable domain which is fused to a T-cell receptor. It emits the need of antigen expressing mechanism to be functional.
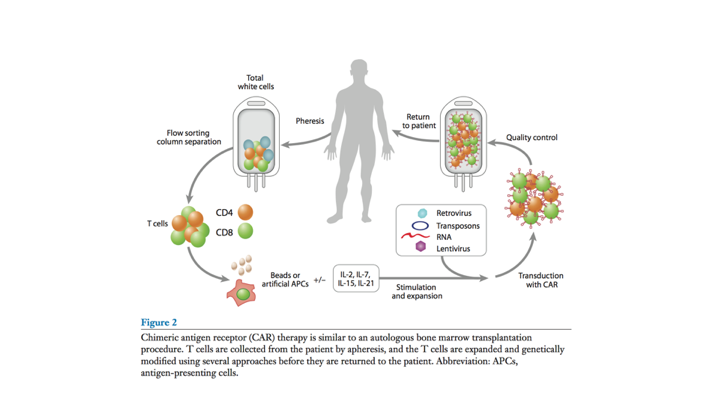
Building a CAR
This is how you build a CAR T. Basically, you have a structurally a single chain antibody that is attached to a T cell or a modified T cell receptor and it targets a native tumor antigen. You then have incorporation of some other stimulatory molecules.
Process of CAR T-cell Therapy
And really how it works is you get a pheresis, you get up regulation of the patient’s T-cells and then you create these chimeric antigen receptors, and then it gets reinfused.
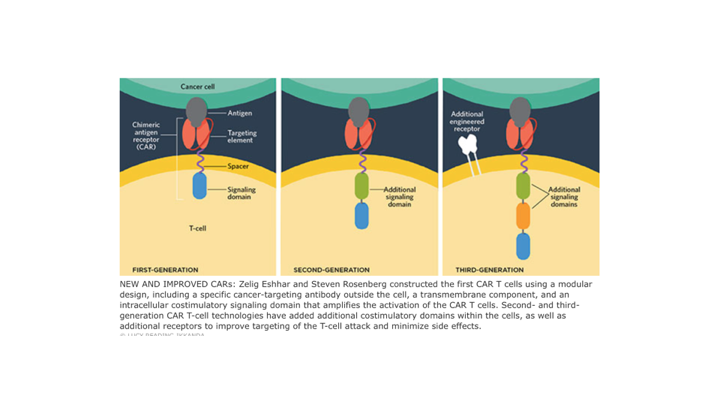
New and Improved CARs
And we have had multiple generations, and again, looking at some various different types, but it all involves basically incorporation of an antibody that is fused to the T-cell receptors.
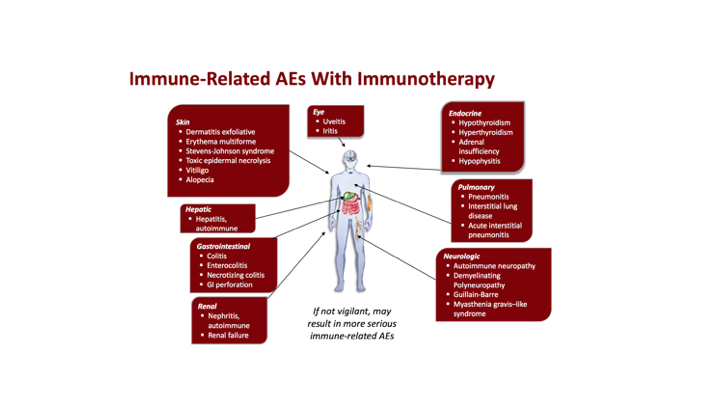
Immune-Related AEs With Immunotherapy
There are some immune-related adverse events with immunotherapy, and again, as we see more and more of these because they are going to be used, they are used right now there’s many of them involved obviously in the bladder cancer space, but you know everything all of these immunotherapies are rapidly expanding with rapid approvals, so we get some dermatitis reactions, some ophthalmologic, some endocrine, and the list and, so again, as we choose, especially the medical oncologists, are really adept at managing these, but as urologists, as we attempt to move more and more of these into our practices, we really, like anything else, need to beware of the side effect profiles with all of these therapies.
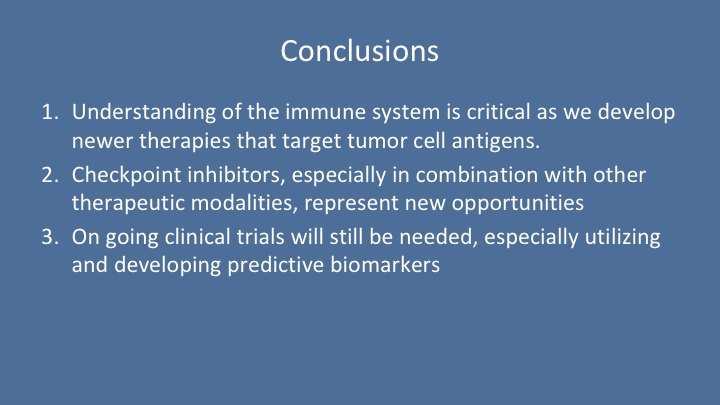
Conclusion
So in conclusion, understanding of the immune system is critical as we develop newer therapies that target tumor cell antigens. Checkpoint inhibitors, especially in combination with other therapeutic modalities, represent new opportunities. Ongoing clinical trials will still be needed, especially utilizing and developing predicted biomarkers. Thank you.
CRAWFORD: Wow, you are smart.
ABOUT THE AUTHOR
Raoul S. Concepcion, MD, FACS, is the Chief Science Officer of U.S. Urology Partners in Nolensville, Tennessee. He was a resident in General Surgery and Urology at Vanderbilt University from 1984-1990, and later served as a Clinical Associate Professor of Urology there.
Dr. Concepcion’s clinical interests revolve around advanced prostate and bladder cancer management. He is a past President of LUGPA. Along with two other urologists, he founded CUSP, a urologic research consortium in the United States. Additionally, he is an advisor and/or speaker for many companies, including Dendreon, Pfizer, Astellas, Amgen, Cellay, and Janssen, and has served as editor for Urologists in Cancer Care.