Michael S. Cookson, MD, MMHC, presented “Integrating the AUA Guidelines into Your Practice” at the International Prostate Cancer Update on January 27, 2018 in Beaver Creek, Colorado.
How to cite: Cookson, Michael S. “Integrating the AUA Guidelines into Your Practice” January 27, 2018. Accessed [date today]. https://dev.grandroundsinurology.com/Integrating-the-AUA-Guidelines-into-Your-Practice/
Summary:
Michael S. Cookson, MD, MMHC, describes how urologists can use American Urological Association (AUA) guidelines to better manage metastatic castration resistant prostate cancer (mCRPC) patients. He stresses the importance of urologists serving as the primary caregiver, the multidisciplinary care model, and organized sequencing of treatments and therapeutics.
(Twitter Question) Reveal the Answer to Audience Response Question #1
A 48 year-old-man with a rising PSA despite a castrate level of testosterone presents for evaluation and possible treatment. A staging workup reveals no radiographic evidence of metastases. In this scenario, FDA approved agents for management include:
- A. sipuleucel-T.
- B. abiraterone + prednisone.
- C. enzalutamide.
- D. docetaxel.
- E. none of the above.
Integrating the AUA Guidelines into Your Practice – Transcript
Click on slide to expand
Integrating the AUA CRPC Guidelines into Your Practice
I would say that three-quarters of the world still isn’t getting this right and hasn’t figured out a way to manage patients with metastatic castrate resistant disease to the best of their abilities with the resources available to them.
Overview
So, my goal is just to talk to you about why urologists should be managing these patients and how to kind of incorporate that multidisciplinary care plan, think about ways to do that in your own practice, review some of the evidence-based therapeutics, try not to overlap, like I’ll not talk about bone health that much because that was covered well a little while ago.
Lesson #1
So my number one message to you is that I think urologists should be the primary caregiver for men with prostate cancer as it relates to that. This includes as they progress through those roller coaster disease spectrums that you have seen on some slides to come and many in the past.
Urologist as the Primary Caregiver
The patient may progress through his disease, but he shouldn’t have to progress through multiple providers and specialists. So, I think we provide a constant to the patient in their life. It’s a real disturbing thing when you have been seeing a patient from diagnosis through treatments, and all of the sudden you tell them you’re not going to see them any more. They know something is really wrong. I think the psychological burden of that alone is a problem. We understand the progression of their disease. We understand the management options, hopefully, of patients, as they progress through those various diseases, and we can coordinate the specialty care that they need from other specialists to treat their cancer as well as lots of ancillary help. So, that is what I’m going to try to outline.
Lesson #2
We need to establish, organize, and manage the multidisciplinary care model that incorporates available resources and works best for your healthcare environment. There is not one size that fits all. So, you have to figure out what it is that you have available to you, and how you can best make that fit.
Multidisciplinary CRPC Clinic
Now, there’s been a lot done on this multidisciplinary care, and urology is late to the table for this, really. But, due to all of the excitement around therapeutics that we have and the increased need to help manage these patients, we know that patients benefit from this, not only from their psyche, and just an overall emotional benefit, but there’s good data from other cancers where they show better outcomes related to the coordination of care, and the better management that happens when experts get together and discuss the options for these patients.
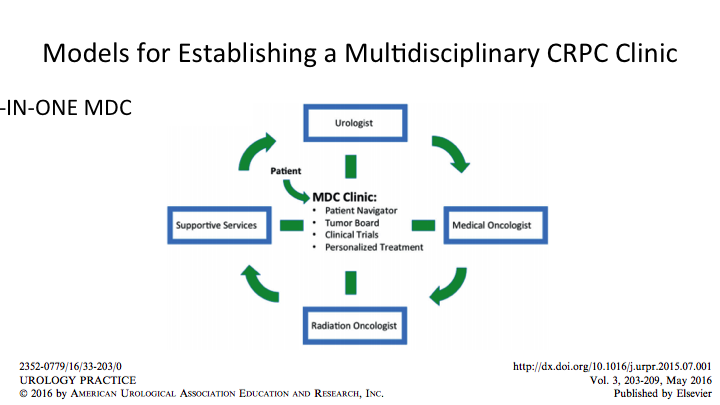
Models for Establishing a Multidisciplinary CRPC Clinic
So, this is a theoretical model that we published, but the concept is there, and you have to, again, kind of figure out how does this work for me when I go home, but the ingredients are the urologist, the medical oncologist, radiation oncology and supportive services, and those supportive services should not be underestimated. Those include navigators or assistants. They can be nurses. They can be PSR. They can be lots of things, but they have to be smart enough to know what is a patient going to need and how do we maximize the visit. Tumor Boards or discussions about what is next for the patient, and how does the patient present, what other imaging or testing does he need? Clinical trials feed right into this, and we wouldn’t have good news to spread in a couple of weeks and all of the FDA approvals came from clinical trials, and so while we’re not curing cancer we are helping people and extending the quality of their life and the extent of their life. We hope to do better in the future, and if we don’t have that coordinated care where we can identify patients for these trials, we won’t be able to move the needle forward. So, I think all those ingredients go together.
Multidisciplinary CRPC Clinic
Now, if you were a patient, you would want the one stop shop, right? All in one, you go to the large group practice, you go to the center, you go to the center, the cancer center, the hospital, you’re going to see the people that you need to see that day. You’re going to block out some time, and you’re going to get as much done in that one period as you can. That is really advantageous for the patient, but not always advantageous for the provider, so that is where that navigation and coordination of care comes in. You don’t want to be sitting in your office while they’re only seeing med onc or radiation oncology and visa versa. So, it has to be worked out so everybody stays busy and it’s an efficient way for you and the patient to be seen.
Multidisciplinary CRPC Clinic
However, we know that one size fits all doesn’t work, and there may not be that option for you available. The patient may not be able to see medical oncology, because they don’t have clinic on the same day you do, or maybe physically you’re not located in the same location. The concept though would continue in an alternative model, which could be a different day if you have to or could be same day different clinic. And I’ll just breeze through that.
Models for Establishing a Multi-Disciplinary CRPC Clinic
It doesn’t really matter. I mean, it’s optimal if you can do it all close in proximity, etc. but the ingredients have to be there if it can’t be done on the same day, and that means communication between providers, some way to share in the patient, whether it’s a virtual tumor board or a real tumor board. Those conversations have to take place in order to maximize the outcome for the patient.
MDC CRPC: Key Ingredients
Again, the ingredients are listed here. I know that many of you who do these know the importance of the navigation, nurse practitioners, and physician assistants cannot be overestimated in this situation. Meetings, depending on availability how often, but more is better than few. If possible, a shared EMR. One of the biggest barriers we have is collating and accumulating all of the information that comes across the fax machine. Again, it lends itself to larger centers or shared EMR if possible. Primary care, now medical oncology may be comfortable managing some of the hypertension and some of the other things that come up, but having a primary care engaged and knowing that they’re receiving the information that you’re doing is also very helpful along with supportive services, and that can be nutritionists, it can be pain management. Certainly palliative care when it is required. So, all of that stuff has to be known, available to you, and you have to be anticipating how you’re going to get them to those next steps.
Lesson #3
The next step is, if you’ve developed this system for managing them, how are you going to deliver the treatments and the therapeutics and organizing your thoughts around the sequencing.
National Comprehensive Cancer Network
There are lots of guidelines out there. They really speak the same language. So, you just have to figure out which one is user friendly for you, and that’s what we tried to do with the AUA guidelines is make them algorithmic or simple because there isn’t really guidance on where it’s appropriate, which one should go first, so that is going to be left to interpretation of the treating physician. But the reality is these things are evidence based. Some have a little bit more to them than others.
American Urological Association (AUA) Guidelines
I’m partial to the AUA guidelines because I think that they were truly evidence based, but they are in constant need of revising, and this year will be no example.
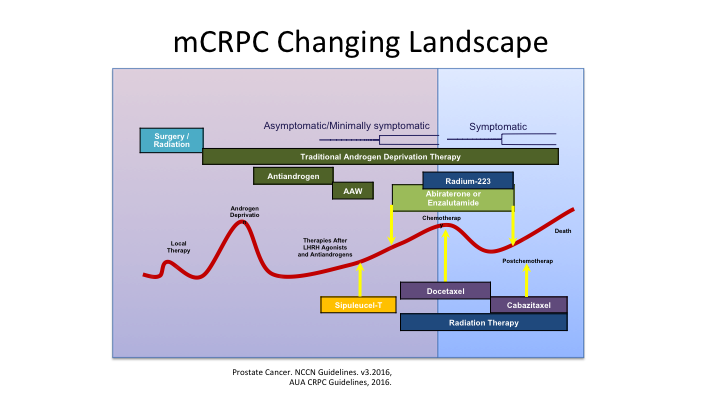
mCRPC Changing Landscape
Dr. Crawford showed this a little bit earlier, and this is that roller coaster slide, which patients don’t like because it is an uphill battle for them as they progress through these various treatments, but when you have all of these different treatments available to you, you have to think in your mind, okay, where is the patient in the journey. Are they hormone naïve, or have they ever seen these types of treatments, are they castrate or endocrine resistant. Do they have metastatic disease or not, and then what are the treatment options available to them?
mCRPC Success Since 1996 Leading to FDA Approvals
There are many treatment options available, the skeletal related, bone health agents were mentioned earlier. Of course those reduce risks of skeletal-related events and can improve on things but they have not extended life. We have certain treatments that are available for pain management including some of the radio nucleotide therapies that were talked about before, except radium, which does extend the life, and then you have survival improving treatments, which are chemotherapies, androgen access as well as the nucleotide therapy of ramucirumab. So all of those are important.
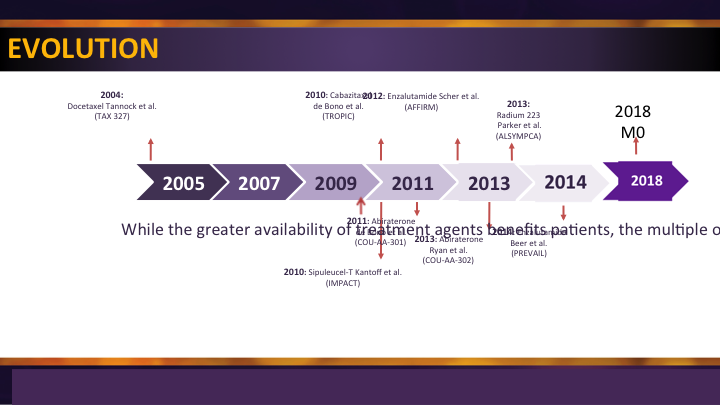
Treatment Evolution
They are all options on the menu. In 2004, docetaxel got approved and things started to happen. There was a drought. About seven years later a second-line chemotherapy and then we started filling in the blanks with some oral agents that were approved post chemotherapy followed by agents that were—the same agents tried in a pre-chemotherapy setting. Radium came along, sip T came along, but we have been a little bit in a drought for a couple of years while we wait for the next step. And while Lenny Gomella said it’s the year of the Eagles, we’ll wait and see about that. I think it is the year of the M0, because now we’re going to start seeing some activity in a space where we’ve had no treatments before. So I’m excited about that.
Index Patients
We, when we made these guidelines, developed them based on partly on the ways the FDA approved these agents, and partly based on the clinical recognition that the presence of absence of metastatic disease was important. The degree and severity of their symptoms was important. Performance status was critical. We can’t give these drugs to everybody, and again, in the beginning when drugs were being approved prior docetaxel was sort of mandated to get the ball rolling, and that has changed a lot over the years.
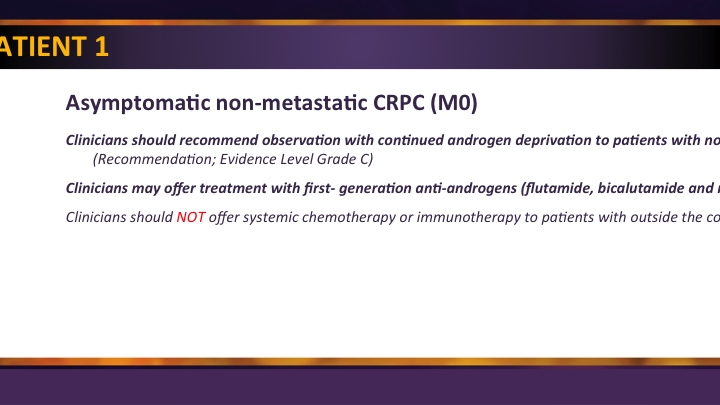
Index Patient 1
This M0 patient which was the ARS question is the unmet need, and 75% of you already knew that we have nothing really approved in this space yet. So things get done for these patients but they are really just kind of manipulating the PSA a little bit and not really extending their life or really changing anything. So we’re excited about this, and this will be updated as soon as the data comes forward, we think, in a couple of weeks we’ll get the presentations, the publications should come pretty quickly thereafter, and then we’ll be in business.
Index Patient 2
For the most common patient who has early metastatic CRPC, those with asymptomatic or minimal symptoms we have a lot of options. So there’s abiraterone with prednisone, enzalutamide, docetaxel. We can give sip T so all of those are options for those patients, and if you’ve been doing some of the things that we’ve talked about like Dr. Crawford was mentioning, really monitoring those patients to see where they are at, you’ll catch them before symptoms are plaguing them and they lose their eligibility for some of these treatments.
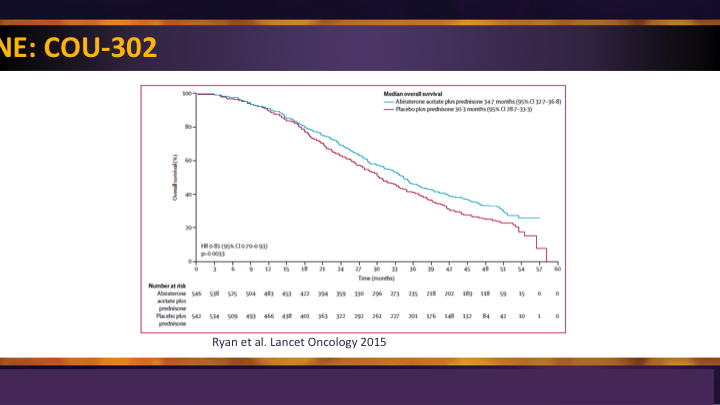
Abiraterone: COU-302
I’ll just briefly say that the COU-302 trial was the second trial for abiraterone plus prednisone, and it did demonstrate not only a survival advantage as well as progression-free survival advantage in patients with metastatic castrate resistant disease prior to chemotherapy.
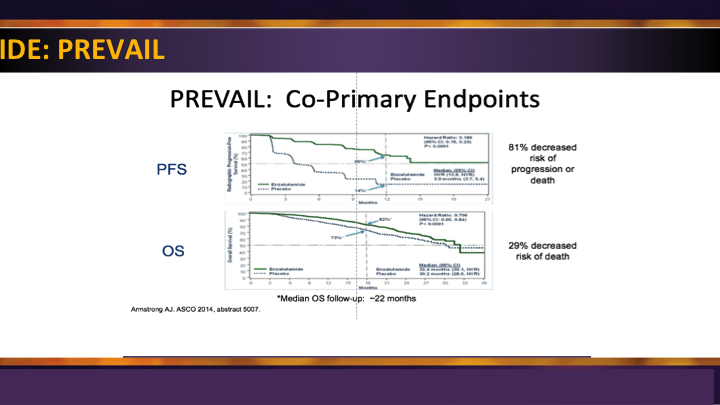
Enzalutamide: PREVAIL
The enzalutamide PREVAIL trial, similarly, was the second big study for them, and it was compared enzalutamide to placebo, again showing significant survival advantage and radiographic progression-free survival in men prior to use of docetaxel.
Enzalutamide: PREVAIL #2
So these were game changers really because before these two trials came out, we waited until they progressed through docetaxel to even begin to give them their treatment.
Docetaxel: TAX-327
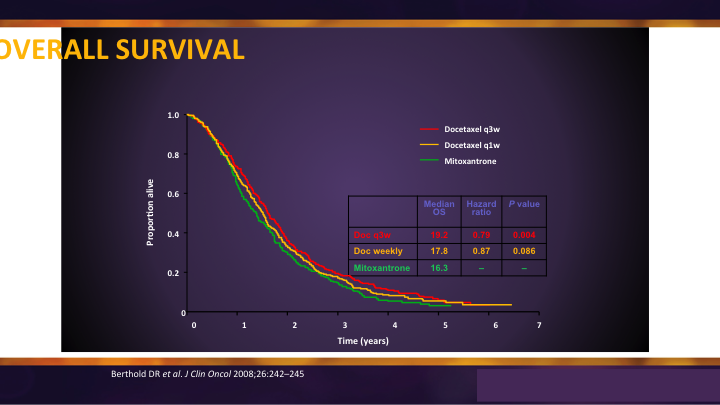
Docetaxel was the original chemotherapy that showed benefit in survival. It was a three-week, every three week regimen, and it was compared to weekly and it was compared to mitoxantrone, and I think sometimes we forget that. It wasn’t compared to placebo. It wasn’t compared to water because when you look at the overall survival you see there is benefit and it translated only into an average somewhere around 3 to 4 months.
TAX-327 Overall Survival
But what would have happened if they had received no chemotherapy, and what would have happened if they received the chemotherapy earlier? Those are questions we’re starting to understand now that docetaxel is being used in an earlier stage in the hormone sensitive area.
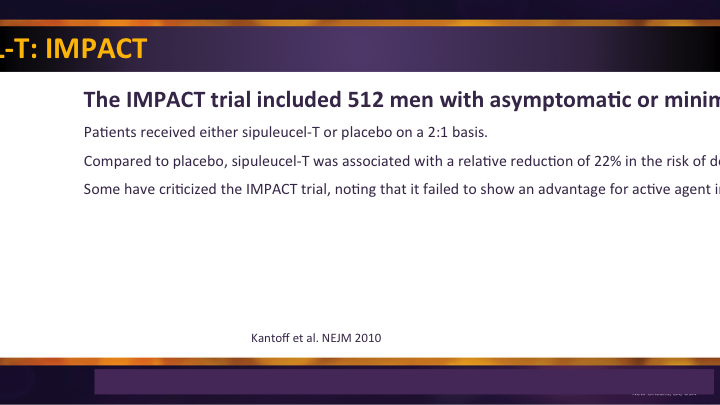
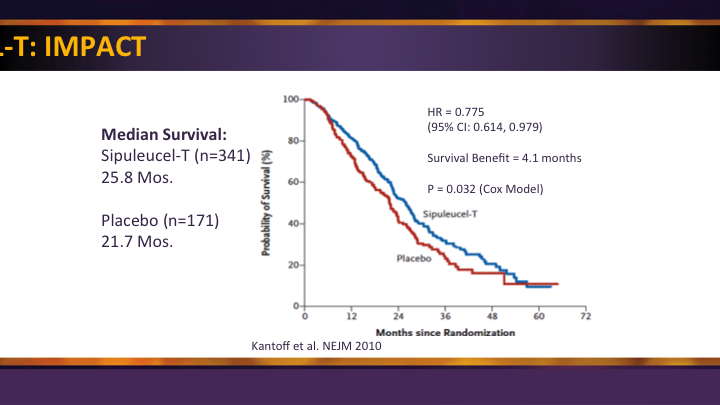
Sipuleucel-T: IMPACT
The use of immune-based therapy, the only immune-based therapy approved for prostate cancer, SIP-T with its IMPACT trial was a significant improvement. They were a little narrow in their approval, so it is really reserved for those minimally symptomatic and asymptomatic patients, and so in that group there was a significant survival benefit.
Sipuleucel-T: IMPACT #2
And so that is where it’s used, but once they determine that they are having symptoms, especially enough for narcotics, it’s really hard. We’re fighting an insurance battle as I speak, because a patient was taking some narcotics for his knee. Had nothing to do with his cancer, but they saw it, and they don’t want him to get approved for the SIP-T we’ve already administered. So, we’re having to go back and explain to them this narcotic wasn’t for his cancer, but it’s an uphill battle when you have that happen.
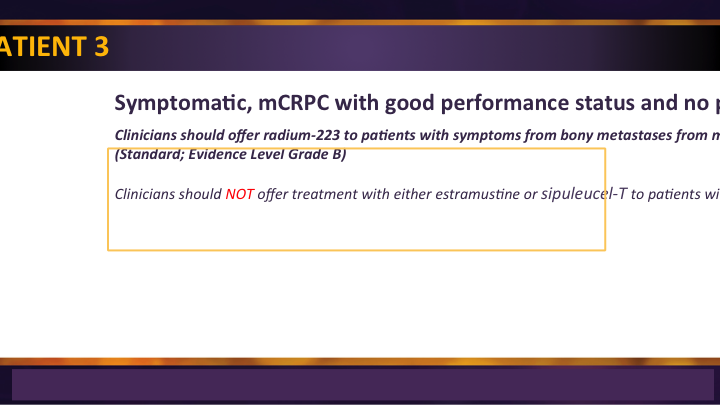
Index Patient 3
Index patient 3 were those patients with symptoms, usually in the studies it was described as pain, but there were many symptoms of metastatic disease. And in there, the same options exist with the exception of SIP-T, which drops off when they’re symptomatic.
Index Patient 3 #2
Additionally, Radium 223 is an option for these patients who have bone lesions, no visceral metastases and it’s approved both in the pre-chemotherapy as well as in the post chemotherapy space.
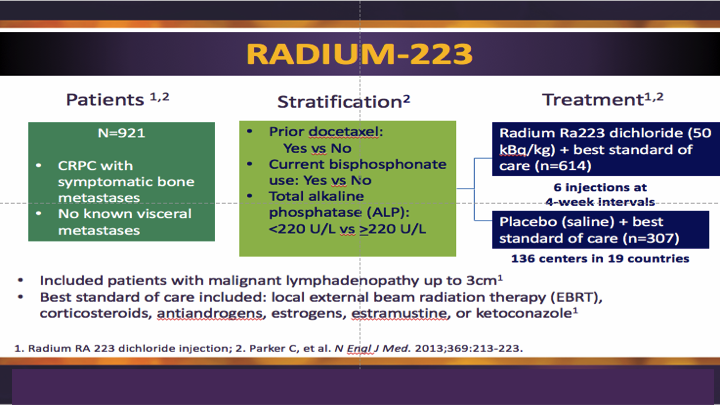
Radium 223
Lenny showed this study earlier, so I don’t have to really go over it except to say that it was best standard of care compared to radium, best standard of care.
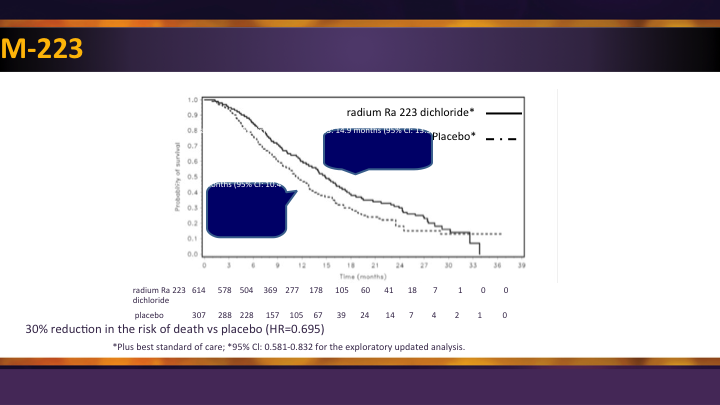
Radium 223 #2
Survival benefit first time seen using radionuclide. All of the other stuff we had before was toxic and palliative, and so this was an advance forward.
Index Patient 5
Index patients 5 are the patients who have seen prior docetaxel chemotherapy. Many of the patients that we’re going to see going forward will have seen docetaxel before.
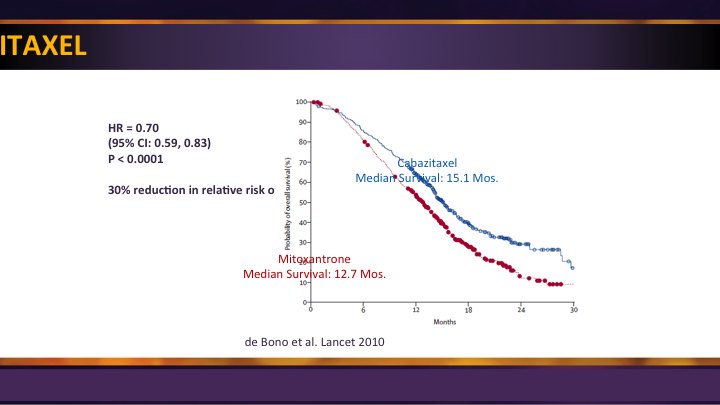
Cabazitaxel
Cabazitaxel is a salvage regimen. Some people are using it up front, and maybe Dr. Petrylak will talk about that, but it got approval as a second-line therapy in that setting.
Cabazitaxel
With again significant survival advantage compared to mitoxantrone post docetaxel so it is an option.
Post Chemotherapy Clinical Trials
And then as I mentioned earlier, abiraterone, enzalutamide, as well as radium plus cabazitaxel, are all options for men who have been through and progressed through the docetaxel.
Future Directions: M0 CRPC
Future directions, really the M0 space, and that is where we’re headed. So, I hope next year, when we’re at this meeting we’ll have lots of good stuff to show you, but right now these are the trials.
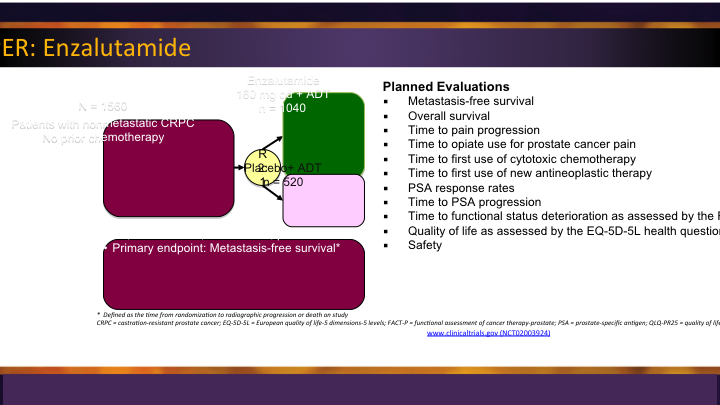
M0 CRPC – PROSPER: Enzalutamide
So most of us are aware that enzalutamide has a trial looking at the M0 space called PROSPER, and it randomized those men with no radiographic evidence, and the outcome that they’re looking for here is metastatic free survival, so that is an important thing, different than overall survival, which was the approval for most of the other drugs that we looked at.
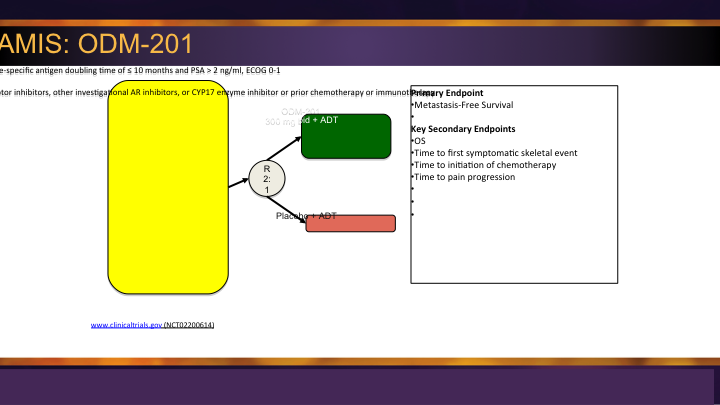
M0 CRPC – ARAMIS: ODM-201
There is another agent, ODM-201, the Aramis trial, which also is in that same space, similarly designed placebo versus the agent gaming for metastatic free survival.
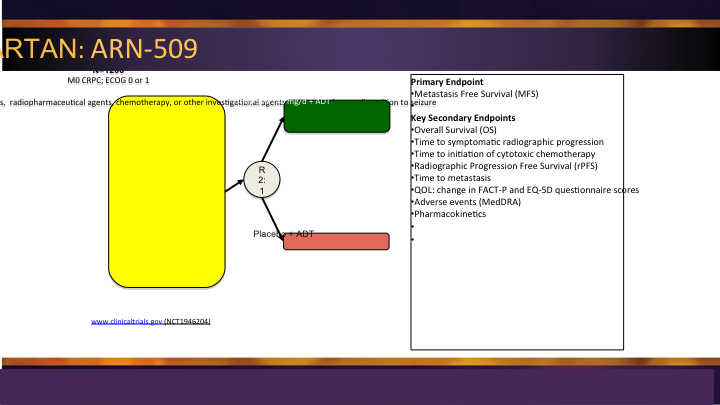
M0 CRPC – SPARTAN: ARN-509
And then finally there is a third one out there, ARN-509 the SPARTAN trial. So three agents all going after that same patient population, all with that metastatic free survival endpoint along with secondary endpoints but it will be way too soon to see any overall survival.
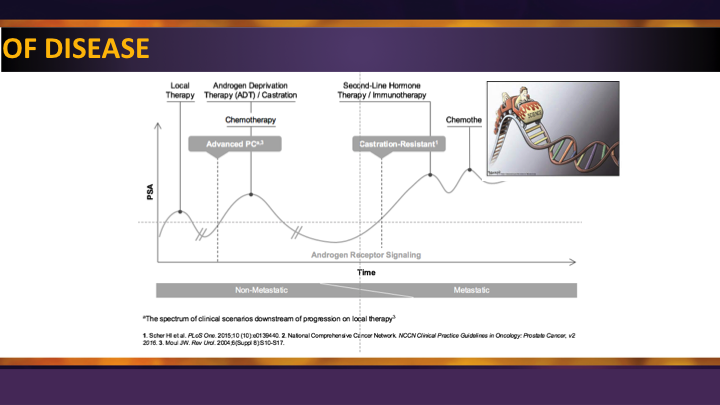
Spectrum of Disease
So the spectrum of disease is shown here, and unfortunately, for patients, they don’t like it. The treatments are moving back, so we started with end stage, post docetaxel, pre-docetaxel, now we’re moving into the M0. We’ve already moved on the other side of the equator, when patients present with newly metastatic disease. When the guidelines get revised by the AUA, there is a bigger move afoot to do more of an advanced prostate cancer guidelines now that we have good data from STAMPEDE, CHAARTED, LATITUDE, in addition to these new agents in the M0 space. So, I think 2018 will be a banner year again for improving the outcome for patients with metastatic castrate-resistant disease. I think that our goal here is to try and make sure as many as we can of the urology large groups, small groups, academic, private, that they understand these treatments, and we’re really the messengers and the primary caretakers of these patients.
Conclusions
So in conclusion, urologists should be the primary caregivers for these men with advanced prostate cancer, establishing multi-disciplinary clinic is an important component to this, and you have to cobble together the resources that are available to you based on your own situation. We have lots of good therapeutics in an area where we had no therapeutics before and lots of things to offer patients. Do they cure them? No. But will they help them? Yes. And when you’re in that situation, you are going to want somebody to help you, too, and these are important advancements for them. And so the guidelines are going to be updated, NCCN I’m sure, the AUA guidelines for sure, and we hope that they will be more robust than what we were able to do the first couple of times, based on what was out there. So, I finished a little on time, which is not a problem around here, and thank you for your attention.
Question from the Audience:
Mike, that was a great presentation. We use the AUA as well as having the NCCN side by side. I think that navigating through the AUA guidelines is much easier, and the reason that the NCCN classically they were quick to respond to new things that are happening. I’m glad to hear you say that you’re going to get something on the M0. And hopefully, you’ll get in the hormone sensitive space with the CHAARTED and the LATITUDE trials to help all the clinicians out. I don’t know, are you going to try to get that on for 2018 also?
Dr. Cookson’s Answer:
The plan is to go in and have as much as we can – – this year. So, I don’t know the publication dates yet, and just presenting something in abstract form won’t be enough to move the needle, so the publications will come, I think, at least two of them soon. We already have enough of the data to do the advanced prostate cancer stuff, so the goal I think is to have something new by AUA time which is early May. So that is the plan. You are right, because of the mechanism in place for the evidence-based data dredge that they do with a company out of the Mayo Clinic, it has been a little slow to react. I find the NCCN is almost like a compendium. If it becomes FDA approved, it like, fits right in the slot. They know they need to be better about it and more nimble, and I have tried to encourage that.
ABOUT THE AUTHOR
Michael S. Cookson, MD, MMHC, is Professor and Chairman of the Department of Urology and holds the Donald D. Albers Endowed Chair in Urology at the University of Oklahoma Health Sciences Center in Oklahoma City. He has authored some 240 peer-reviewed journal publications as well as more than 30 chapters of various textbooks, and he is nationally recognized for his outstanding contributions to urologic oncology. Dr. Cookson completed his Urology Residency at the University of Texas, San Antonio, and completed his Urologic Oncology Fellowship at Memorial Sloan-Kettering Cancer Center in New York. From 1998 to 2013, he served as the Vice Chairman of Urologic Surgery and Director of the Urologic Oncology Fellowship Program at Vanderbilt University. Dr. Cookson has devoted much of his academic career to the management of patients with urologic cancers, with a strong emphasis on clinical guidelines, education, and evidenced-based medicine. He was a member of the AUA/ABU Examination Committee for 10 years, serving as Oncology Consultant and Pathology Editor. He also serves on the ABU Oral Examination Committee. He is a Co-Founder of the Oncology Knowledge Assessment Test (OKAT), an SUO-mandated examination. He also served as Chair for the OKAT for 5 years. In 2011, he received the President’s Distinguished Service Award from the SUO for educational contributions. He received the 2018 AUA Presidential Citation for Outstanding Service for his role in the development of the OKAT and as Chair of the Castration-Resistant Prostate Cancer Guidelines Committee at the AUA 2018 Annual Meeting. Dr. Cookson has previously served as a member of the AUA Guidelines on Localized Prostate Cancer Committee. Dr. Cookson is currently serving out the 2019-2020 term as the SUO President.