A. Karim Kader, MD, PhD, presented “Is MRI Fusion Biopsy the New Gold Standard of Diagnosis? Pro Argument” during the 28th Annual Perspectives in Urology: Point Counterpoint on November 15, 2019 in Scottsdale, Arizona.
How to cite: Kader, A. Karim. “Is MRI Fusion Biopsy the New Gold Standard of Diagnosis? Pro Argument” November 15, 2019. Accessed Jan 2025. https://dev.grandroundsinurology.com/is-mri-fusion-biopsy-the-new-gold-standard-of-diagnosis-pro-argument/
Is MRI Fusion Biopsy the New Gold Standard of Diagnosis? Pro Argument – Summary:
A. Karim Kader, MD, PhD, argues that MRI-guided biopsy followed by a systematic biopsy should be the gold standard in prostate cancer diagnosis. He reviews the PROMIS and PRECISION trials to compare prostate cancer detection with MRI guidance versus systematic and transrectal ultrasound-guided biopsy.
The “con” rebuttal to this presentation is “Is MRI Fusion Biopsy the New Gold Standard of Diagnosis? Con Argument” by E. David Crawford, MD.
Abstract:
Historically, prostate biopsies involved digital guidance along with systematic biopsy. Even with the introduction of transrectal ultrasound (TRUS), there was an unmet need in prostate visualization to guide biopsies.
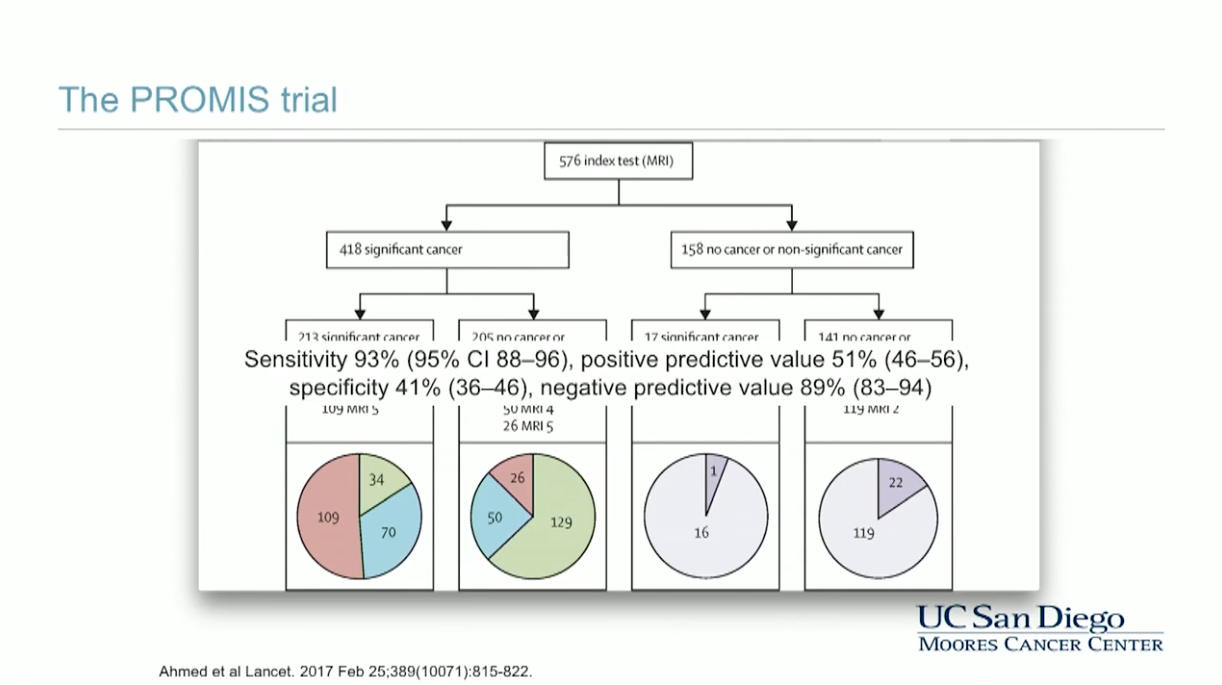
In 2017, the PROMIS trial demonstrated that using multiparametric MRI as an initial triage for men with an elevated PSA could allow 27% of patients to avoid a primary biopsy and diagnose 5% fewer clinically insignificant cancers. When compared to using a standard TRUS-guided biopsy pathway, using multiparametric MRI to guide biopsy can allow urologists to detect 18% more cases of significant cancers.
Furthermore, when observing the concordance between biopsy and radical prostatectomy pathology, multiparametric MRI-guided biopsy better predicted cancer grade than did systematic biopsy.
Famously, in the PRECISION trial, MRI with or without targeted biopsy detected clinically significant cancer in 38% of patients, as compared to standard biopsy, which detected clinically significant cancer in 26% of patients in a separate arm. Also, fewer patients received a diagnosis of clinically insignificant cancer in the MRI-targeted biopsy group compared to the standard biopsy group.
Because of these results, it can be argued that not offering this technology to patients is unjustifiable.
About Perspectives in Urology: Point Counterpoint
Perspectives in Urology: Point Counterpoint (PCP)is an annual, multi-day, CME-accredited conference devoted to discussing and debating the latest topics in men’s health and general urology, as well as management of bladder, renal, and both localized and advanced prostate cancer. More than didactic lectures, the conference’s format includes debates, point-counterpoint discussion panels, and unique case-based presentations. Dr. Kader presented this lecture during the 28th PCP in 2019. Please visit this page in order to register for future PCP meetings.
ABOUT THE AUTHOR
A. Karim Kader, MD, PhD, is a board-certified urologist who specializes in screening, detecting, treating and preventing prostate cancer. As a Professor in the Department of Urology and Director of Urologic Oncology at the University of California, San Diego, Dr. Kader instructs medical students, residents and fellows at UC San Diego School of Medicine. His current research interests include genetic markers to assess risk and predict outcomes in urologic cancer. In addition, he examines the impact of augmented reality and enhanced imaging techniques for education and improved surgical outcomes. Dr. Kader completed a Fellowship in Urologic Oncology at the University of Texas MD Anderson Cancer Center in Houston and a Residency in Urology at the University of Toronto in Ontario, Canada. He earned his medical degree and doctoral degree from the University of British Columbia in Vancouver, British Columbia. Dr. Kader is nationally recognized for his expertise in performing robot-assisted radical cystectomy and urinary diversion for patients with bladder cancer. He holds several patents for genetic discoveries focused on the early detection and prevention of prostate cancer in addition to device patents for prostate cancer treatment. He is the principal investigator in numerous clinical research projects and has published extensively.