Daniel P. Petrylak, MD, presented “New Targets for Advanced Bladder Cancer” at the 2nd Annual International Bladder Cancer Update on January 27, 2018 in Beaver Creek, Colorado
How to cite: Petrylak, Daniel P. “New Targets for Advanced Bladder Cancer” January 27, 2018. Accessed Dec 2024. https://New-Targets-for-Advanced-Bladder-Cancer/
Summary:
Daniel P. Petrylak, MD, discusses the efficacy of checkpoint inhibitors in previously cisplatin treated metastatic urothelial carcinoma patients and the need for better biomarkers for checkpoint proteins. He also identifies future directions for treating the disease, including ipilimumab/nivolumab and epacadostat/ pembrolizumab combinations, and PD-L1 immunohistochemical staining.
New Targets for Advanced Bladder Cancer – Transcript
Click on slide to expand
Alan Egoda
Alan Egoda was really one of the premiere GU oncologists of the 1980s, and I think it’s kind of a tribute to him that it’s taken us so long to come up with new treatments for this disease. We really went about 15 years actually about 20 years until we developed new treatments since the development of M-VAC. Let me just move ahead here.
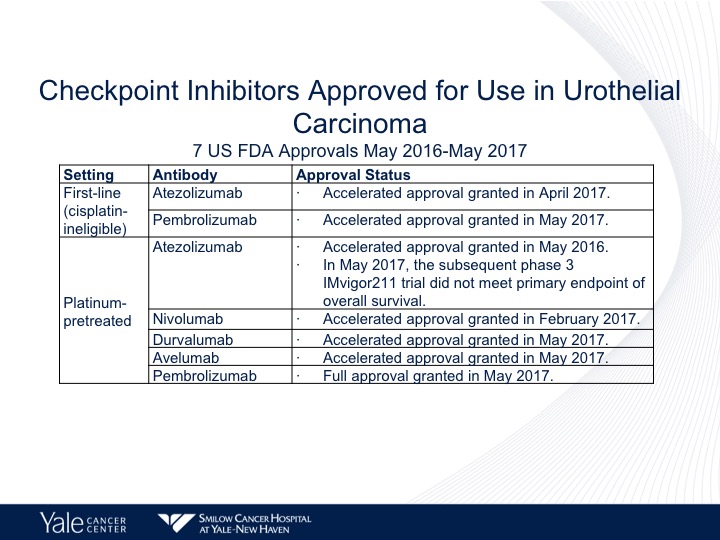
Checkpoint Inhibitors Approved for Use in Urothelial Carcinoma
So checkpoint therapy is approved in the United States for both platinum ineligible as well as platinum eligible urothelial carcinoma. Atezolizumab and pembrolizumab are approved by the FDA front-line. This was granted accelerated approval in May of 2017. We have five checkpoint inhibitors approved for second-line therapy including atezolizumab, nivolumab, durvalumab, and avelumab. And pembro, the only one that has full approval is pembro and that is based upon a randomized phase 3 trial.
Approvals: First-line, Cisplatin-ineligible
I’m just going to quickly go into the fist two approvals for platinum ineligible disease, atezolizumab, and pembrolizumab.
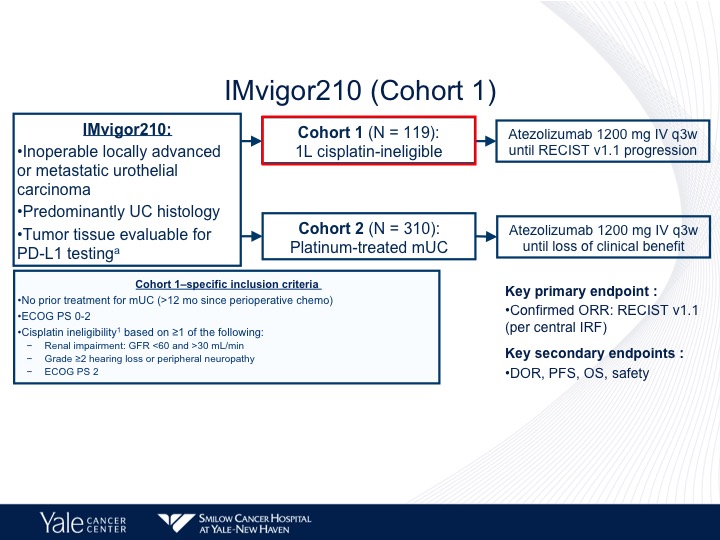
IMvigor210 (Cohort 1)
The IMvigor210 trial was a large Phase 2 trial that had two different cohorts, the first of which was cisplatin-ineligible patients, and the criterion included no prior treatment with chemotherapy for metastatic urothelial carcinoma, ECOG 02 or renal impairment grade 2 or 2 hearing loss or peripheral neuropathy or greater and ECOG performance status of 2.
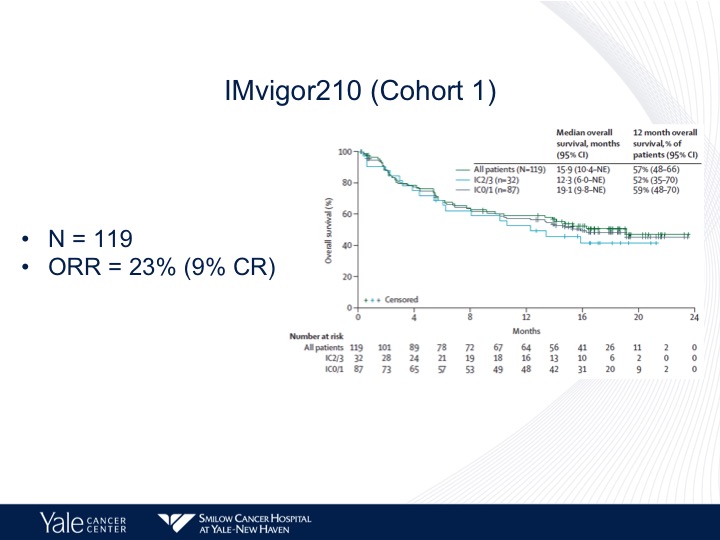
IMvigor210 (Cohort 1)
So when we look at the cohort 1 patients, 119 patients objective response rate of 23% survival of approximately 15 months, we see a disconnect between PD-L1 expression and overall survival. In other words it does not correlate in this particular dataset. That’s different from what we see in the second-line setting.
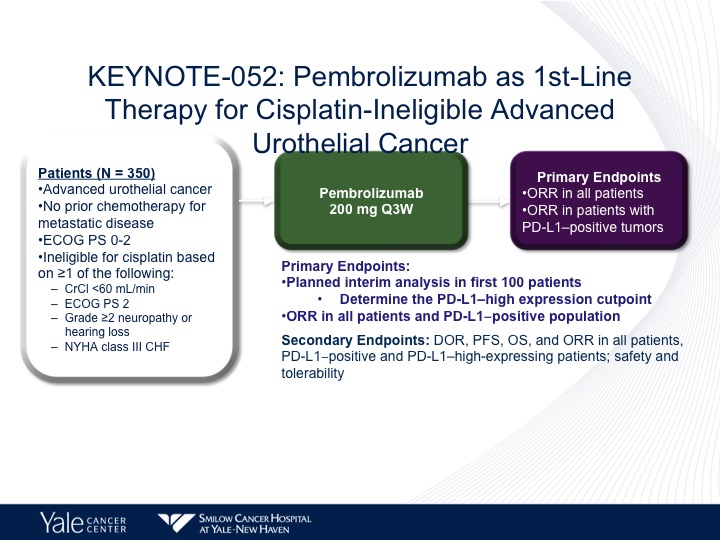
KEYNOTE-052: Pembrolizumab as 1st-Line Therapy for Cisplatin-Ineligible Advanced Urothelial Cancer
The KEYNOTE-052 trial got pembrolizumab approved in the same setting, the difference being that the only other criteria for this drug was New York Heart Association Class 3 or 2 congestive heart failure. And pembro was given at 200 mg every three weeks.
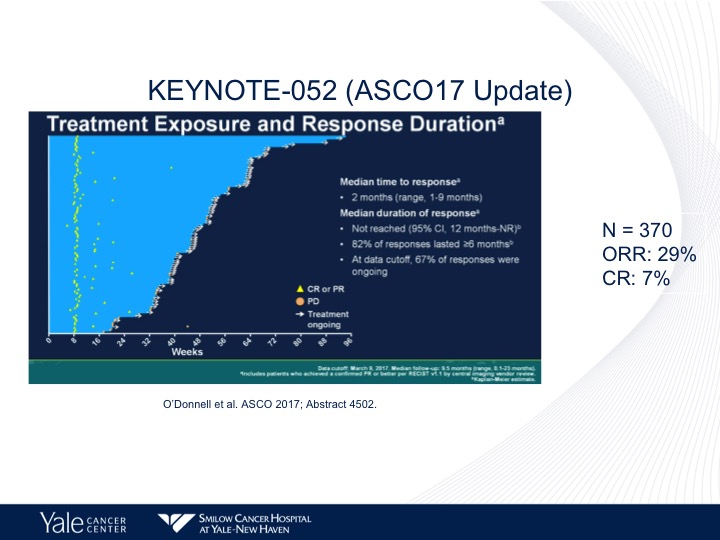
KEYNOTE-052 (ASCO17 Update)
And again the same response rate, objective response rate of approximately 23%. Complete response rate of 7%, and no survival data has yet been reported.
Approvals: Previously-treated Disease
Five drugs as I mentioned before approved in second-line therapy, atezolizumab was the first to be approved based upon the second cohort of the IM.
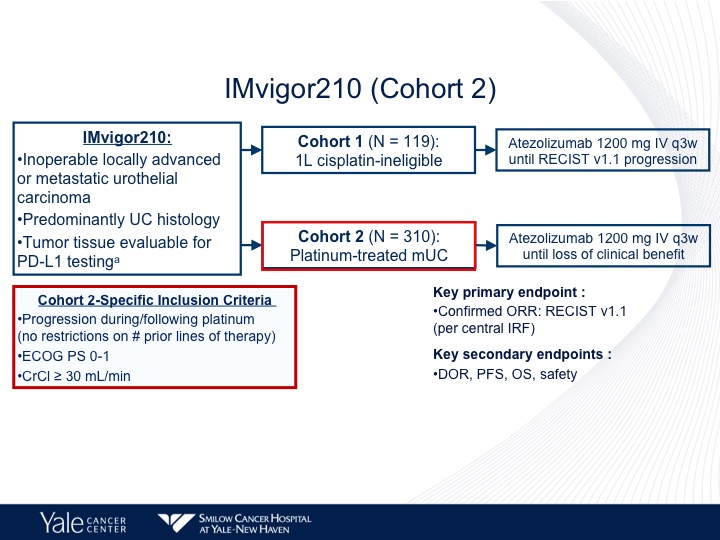
IMvigor210 (Cohort 2)
310 patients with platinum-treated disease and they had to have progression, had to be within two years of the initial therapy, ECOG performance status of 0 or 1, and a creatinine clearance of greater than 30.
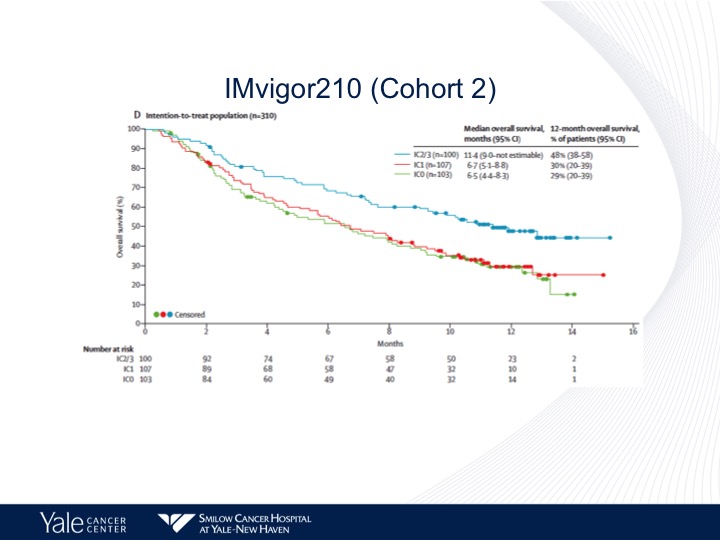
IMvigor210 (Cohort 2)
In contrast to what I mentioned before with the patients who were platinum ineligible, we do see a correlation with PD-L1 expression. There’s greater survival in those patients who have high levels compared to those patients who have no expression or low levels of expression.
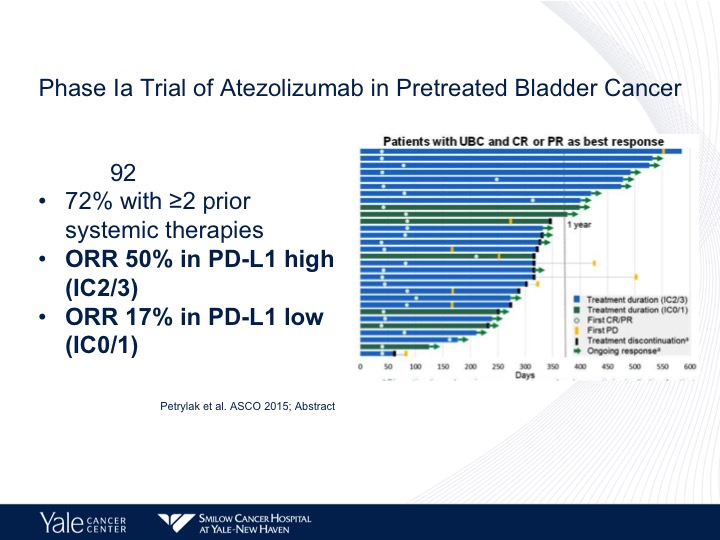
Phase 1a Trial of Atezolizumab in Pretreated Bladder Cancer
We still do see responses in those patients who are PD-L1 negative. It’s about 11%. We do have long-term follow-up data, and this is actually going to be published in JAMA Oncology within the next couple of weeks. This was presented at ASCO GU last year. This is from our Phase 1 experience, and what we found was we looked at the Phase 1 data, and we found that the objective response rate was 50%. It actually was up higher than it was on the initial report and in the low patients it’s 17%.
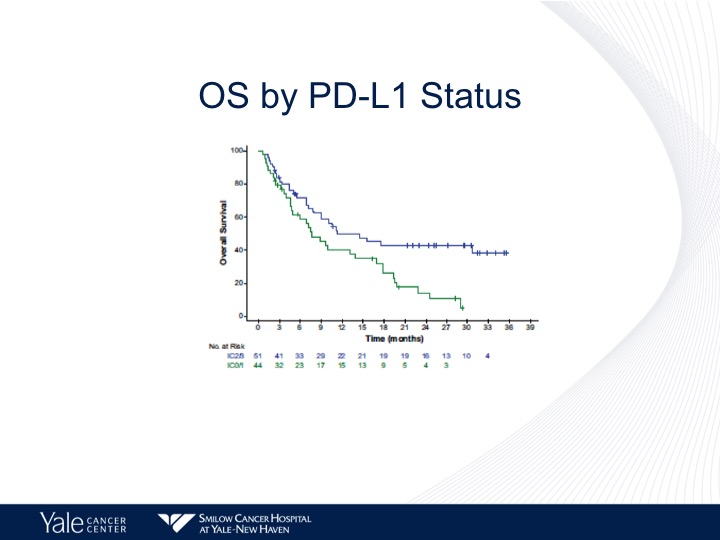
OS by PD-L1 Status
And again a better survival by PD-L1 Status of about 10 months for those patients who have a PD-L1 high versus about 8 months for those patients who had a lower level of PD-L1 expression.
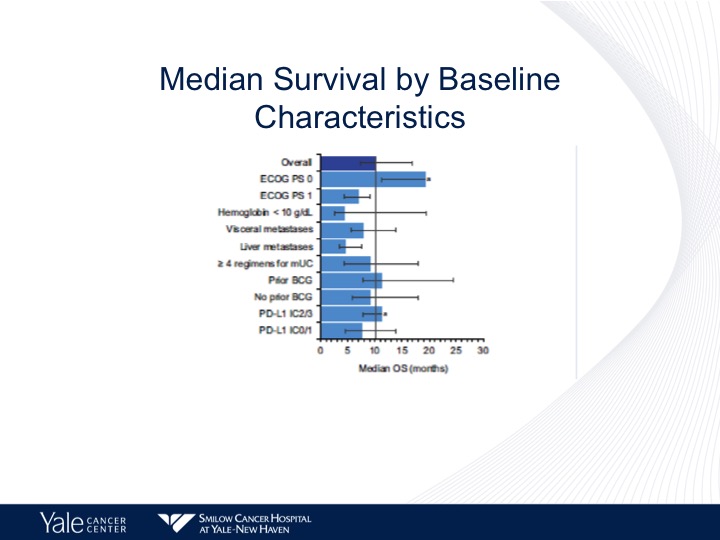
Median Survival by Baseline Characteristics
Now, when we start looking at baseline characteristics, as I mentioned in the previous talk, the one group that does not seem to do as well with checkpoint inhibition therapy are those patients with hepatic metastases. These are primarily patients with good performance status and predominantly nodal disease.
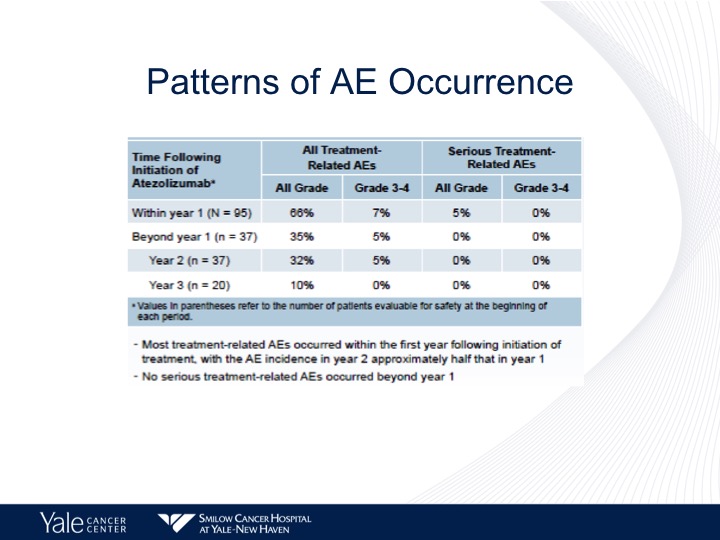
Patterns of AE Occurrence
Now, these patients are treated until progression, and I think it’s important to note that the majority of the side effects are seen within the first year of treatment. We start seeing these diminish over time, and no serious adverse events are reported past year one.
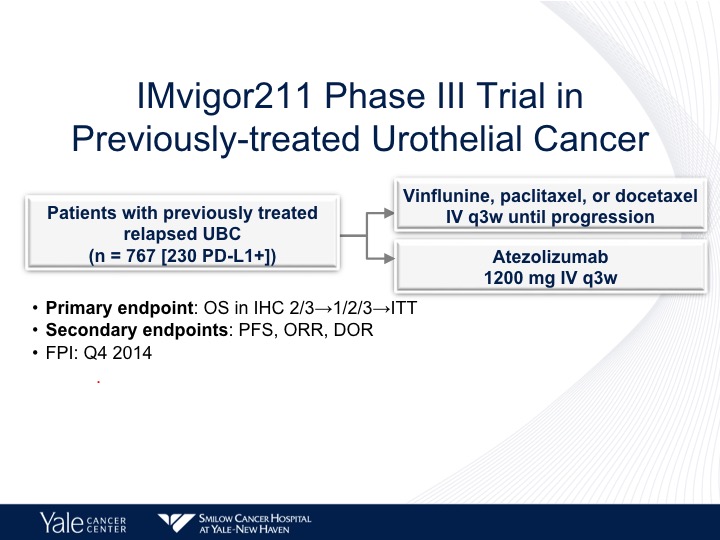
IMvigor 211 Phase III Trial in Previously-treated Urothelial Cancer
Now the IMvigor 211 trial was supposed to confirm these observations. This was run predominantly in Europe by Tom Pals in a randomized 767 patients to chemotherapy with vinflunine in Europe. It was docetaxel and paclitaxel in the United States, and this was compared to atezolizumab. Now, the trouble with this trial is the design.
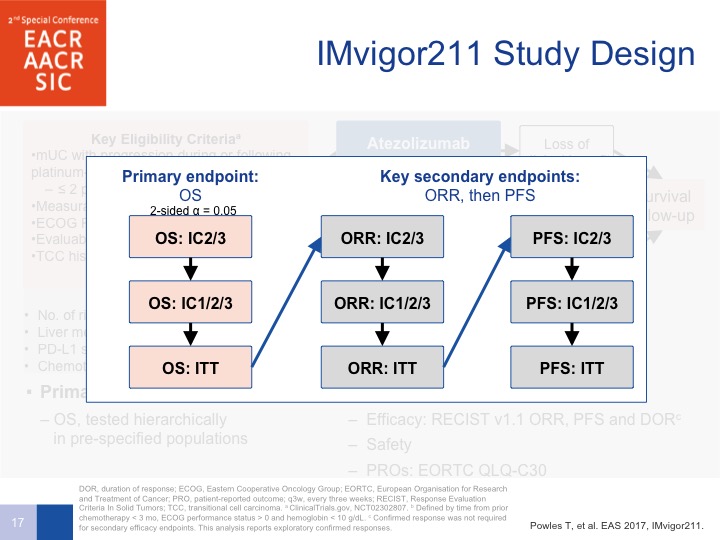
IMvigor 211 Study Design
We were all convinced because of the phase 2 data that the PD-L1 expression correlated with a better outcome as far as survival was confirmed. So the stratified method was designed to have a lower accrual or at least need fewer patients for the exact, the result that we wanted to be seeing, which was a survival benefit. So if, in the PD-L1 positive patients, the primary endpoint was met, which was overall survival, you could proceed on to all patients, or patients who were PD-L1 negative as well. So it was intent to treat and PFS by intent-to-treat but you had to be positive to go onto these two next areas for overall survival of patients, all patients treated on the study.
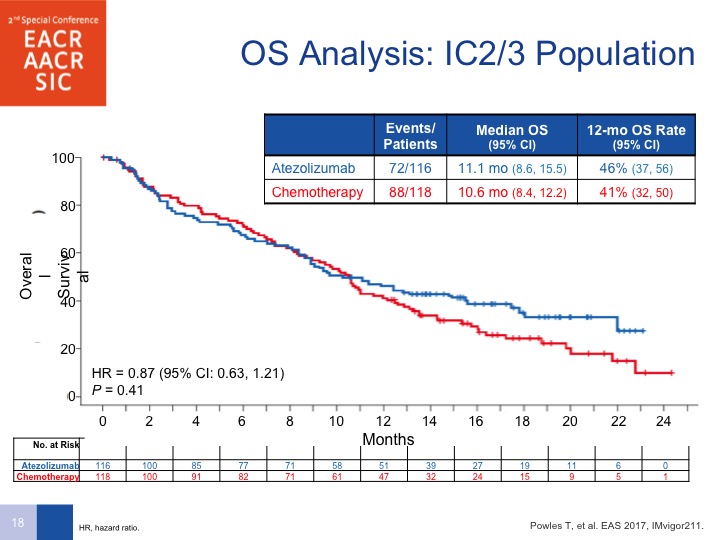
OS Analysis: IC2/3 Population
And lo and behold, when you look at the IC2/3 population, you don’t see a difference. A couple of things are important to point out here. Number 1, as was mentioned earlier, PD-L1 can be predictive for response but it is also a prognostic factor, so it could be that the high levels of PD-L1 portended for a better prognosis, and that is the reason why a survival benefit was not seen. Secondly, this was predominantly vinflunine as far as chemotherapy was concerned, but look at the survival on the chemotherapy arm. Normally we see about a 7 to 8-month survival with chemotherapy, so that is actually better than what you would have expected so there is something a little bit fluky about this design that may have led to the fact that this was not showing any difference in survival.
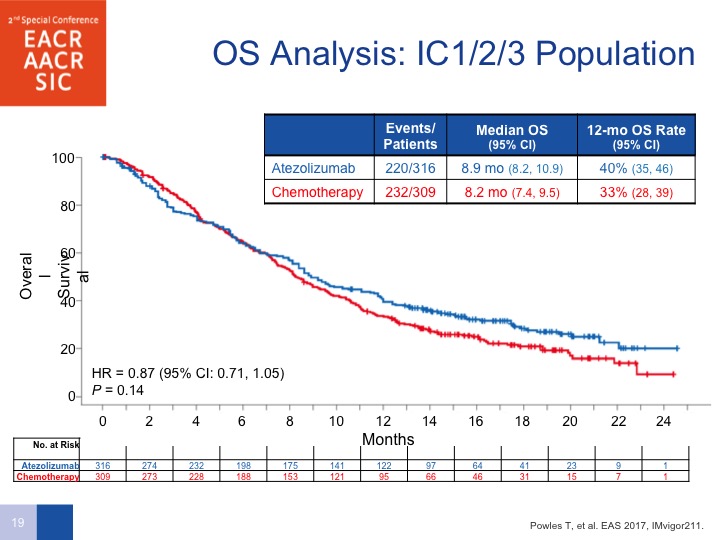
IC1/2/3 Population
If you look at the overall survival analysis by all comers, there does seem to be a difference. Had the trial been powered to do this properly, they would have had a hazard ratio of 0.85 and the P value was 0.038.
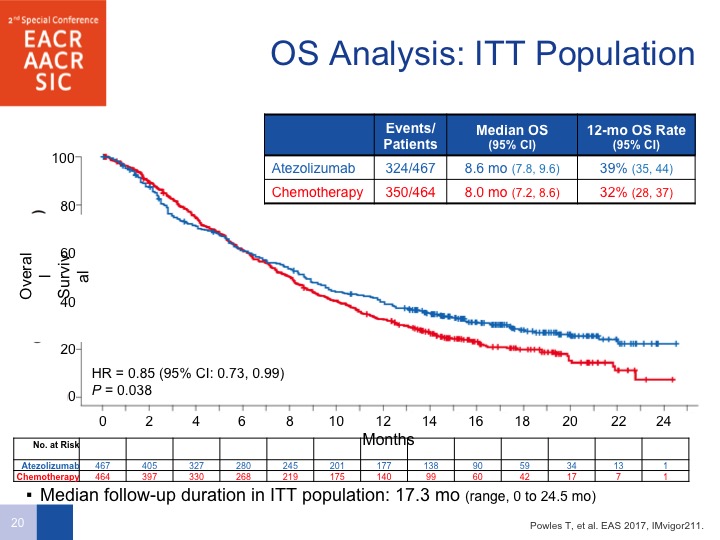
ITT Population
So this is unfortunate, and although do I personally believe there are differences in the checkpoint inhibitors, the answer is no. But the only one that does have a difference or Phase III positive study is pembrolizumab. We’ll get to that data in a few moments.
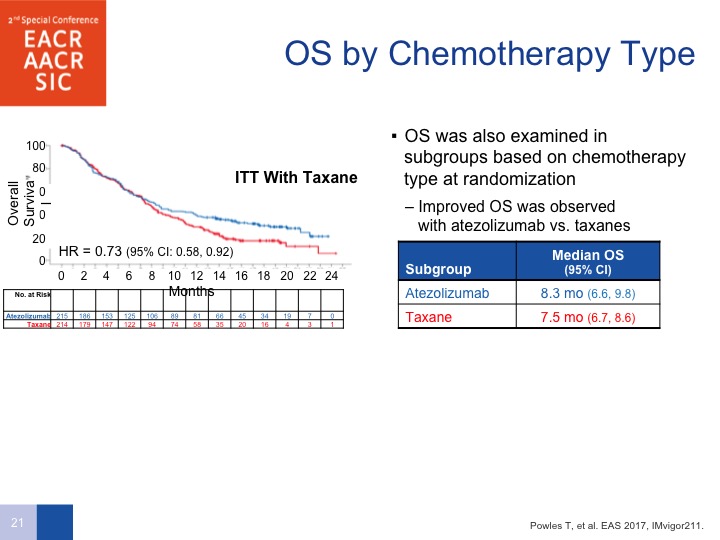
OS by Chemotherapy Type
By chemotherapy type, taxanes, there does seem to be a difference in survival with taxane therapy compared to PD-L1.
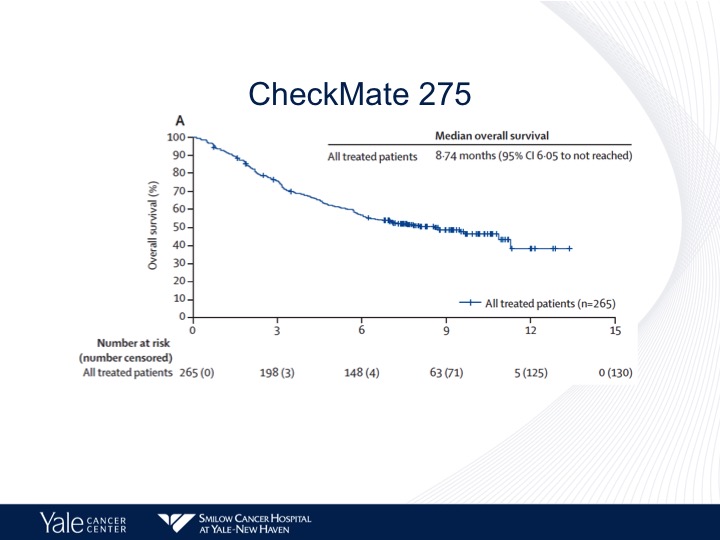
CheckMate 275: Study Design
So CheckMate 275 looked at nivolumab. I’ll go through this very, very quickly.
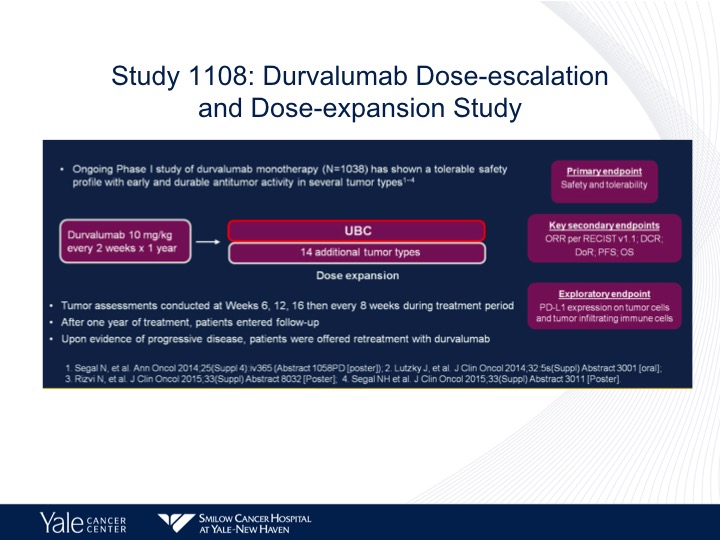
Study 1108: Durvalumab Dose-escalation and Dose-expansion Study
Durvalumab, another phase 1 trial in metastatic urothelial carcinoma given every other week for a year, and again response rates paralleling what we’ve seen before. Maybe a little bit better of a median survival of 18.2 months, but again that could be related to patient selection. There was a correlation between overall survival and PD-L1 expression with this study.
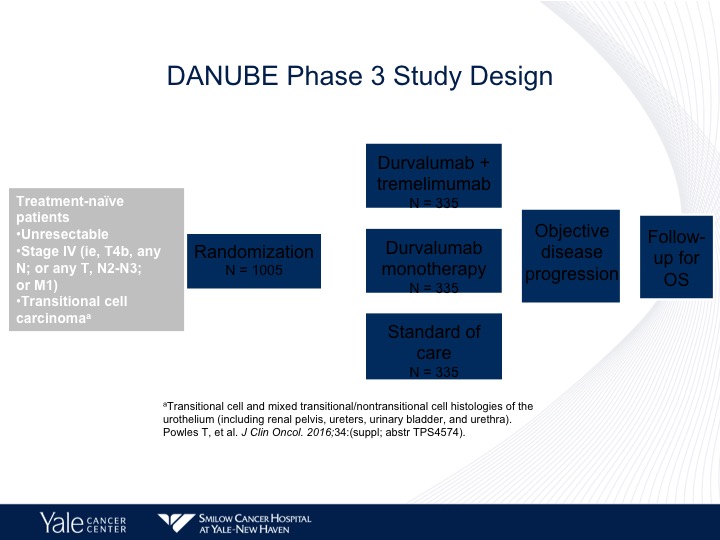
DANUBE Phase 3 Study Design
The DANUBE study is taking avelumab up front, and it’s comparing chemotherapy to avelumab plus tremalulumab or avelumab alone. The trial is accrued, and we hopefully will have an answer very, very shortly as to survival.
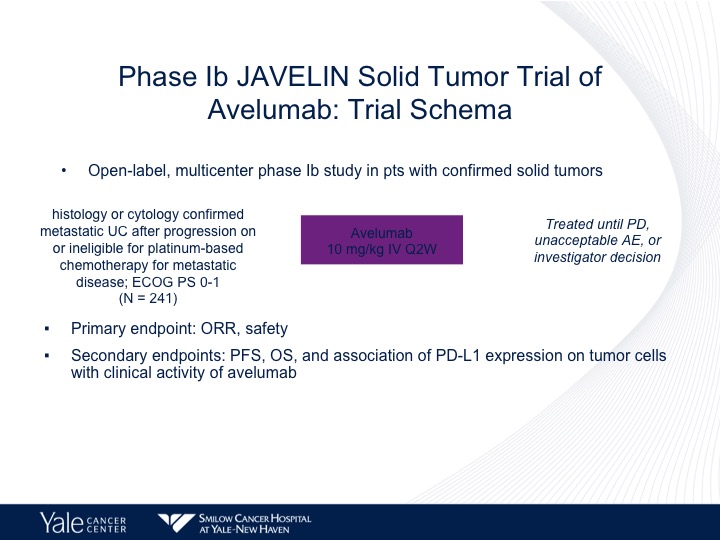
Phase 1b JAVELIN Solid Tumor Trial of Avelumab
Avelumab has also been evaluated in a Phase 1 trial, the JAVELIN study.
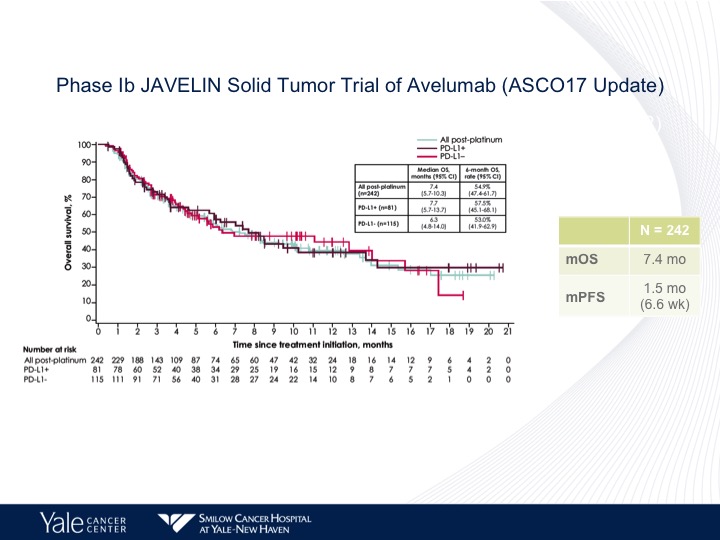
Phase 1b JAVELIN Solid Tumor Trial of Avelumab (ASC017 Update)
And again no difference in the outcome as far as PD-L1 status and similar survival as to what we have seen before.
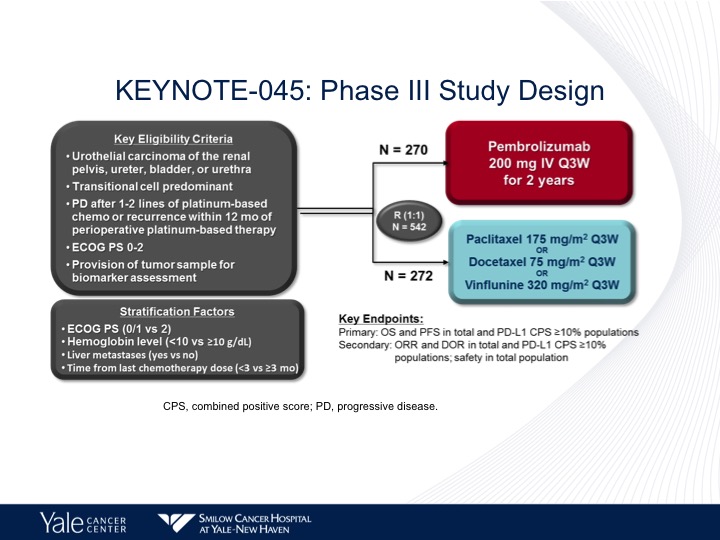
KEYNOTE-045: Phase III Study Design
As I mentioned before, the pembrolizumab is the only checkpoint inhibitor that shows a difference in survival in a Phase 3 trial. Almost identical numbers of patients to what we saw with the atezolizumab study. The same control arm paclitaxel/ docetaxel or vinflunine.
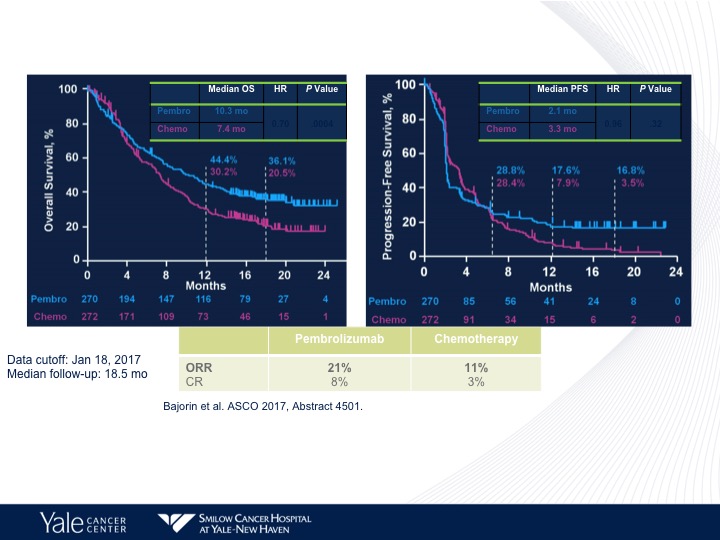
Comparing Pembrolizumab to Chemotherapy
And here we do see a survival benefit again in favor of pembrolizumab compared to chemotherapy. The chemotherapy arm is more akin to what we would see with chemotherapy in this situation. Also a better objective response rate with pembrolizumab compared to chemotherapy, but no difference in progression-free survival between the two different arms. That is again a theme we see with immunotherapy.
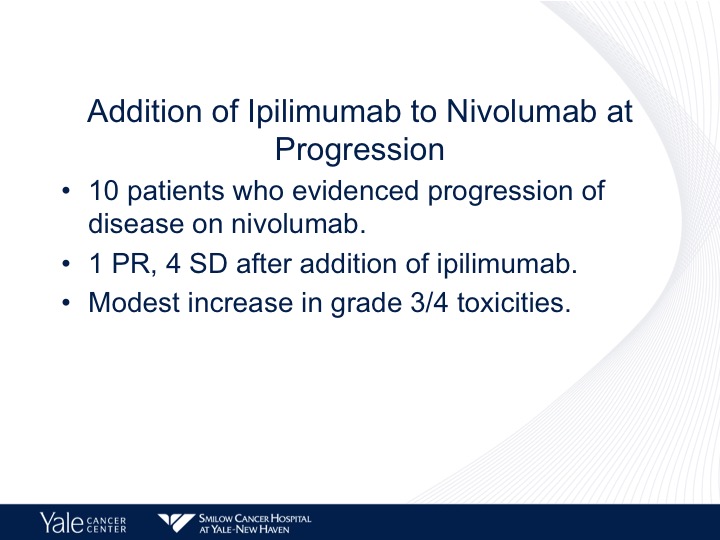
Addition of Ipilimumab to Nivolumab at Progression
Well as was said in the first talk, adding ipilimumab to nivolumab is one way this can come about. There is no specific phase 2 trial that has as yet been published, but there have been studies that have added ipilimumab at progression and in 10 patients this really is very, very small but one partial response was seen.
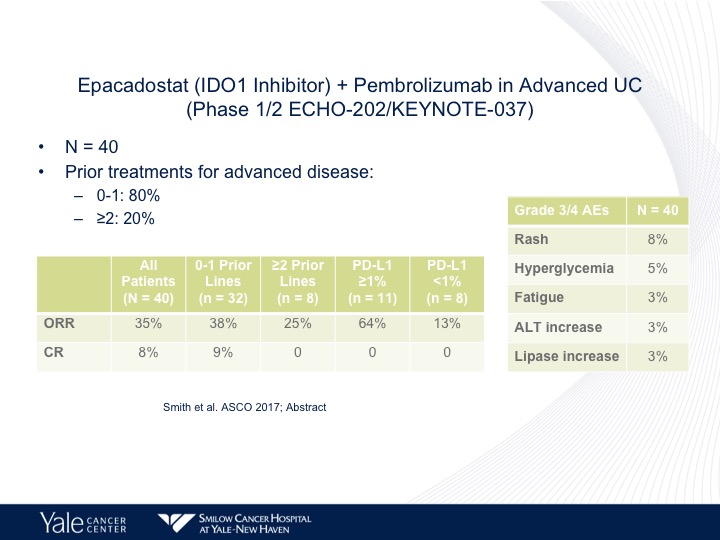
Epacadostat (IDO1 Inhibitor) + Pembrolizumab in Advanced UC (Phase ½ ECHO-202/KEYNOTE-037)
What I think is a very interesting area of investigation is epacadostat, which is a inhibitor of a tyrosine metabolism. This actually may reverse some of the mechanisms of what is called IDO or IDO inhibition. That’s a metabolite that will cause immune resistance and a small trial by Dave Smith, there does seem to be a higher response rate when epacadostat is combined with pembrolizumab, and this is now going into randomized trials both up front in platinum ineligible as well as in second line platinum eligible patients.
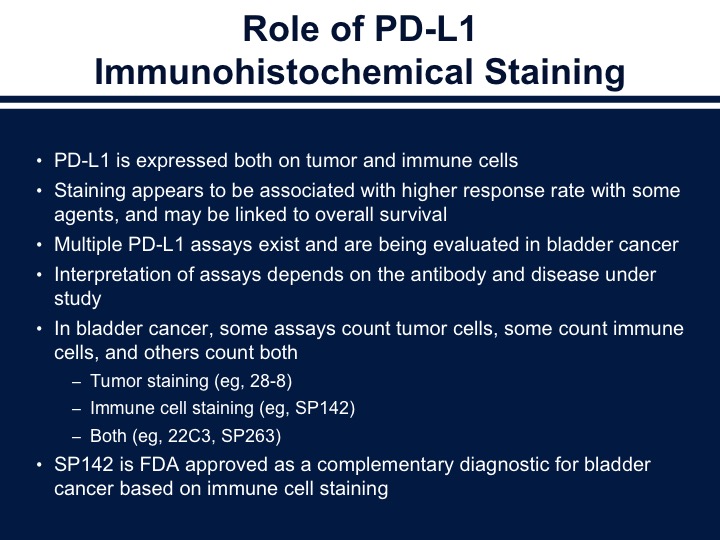
Role of PD-L1 Immunohistochemical Staining
So we talked a little bit before about markers and the issue about markers I think is difficult to really interpret because of the fact that we’ve got different antibodies. These recognize both tumor cells as well as the immune cells, and we do see a correlation between response and the overall outcome. I think my biggest critique of these studies is they don’t use consistent collection of tissue, and if we’re talking about an acute phase reactant or an immune treatment. This may change over time or change after treatment. So again, there are different assays. They look at different portions of the stroma as well as the tumor, and as you see on the bottom the tumor staining is the 28-8 antibody, the SP142, which is the new Genentech antibody, only looks at the immune cells and then both the 22C3 and the SP263.
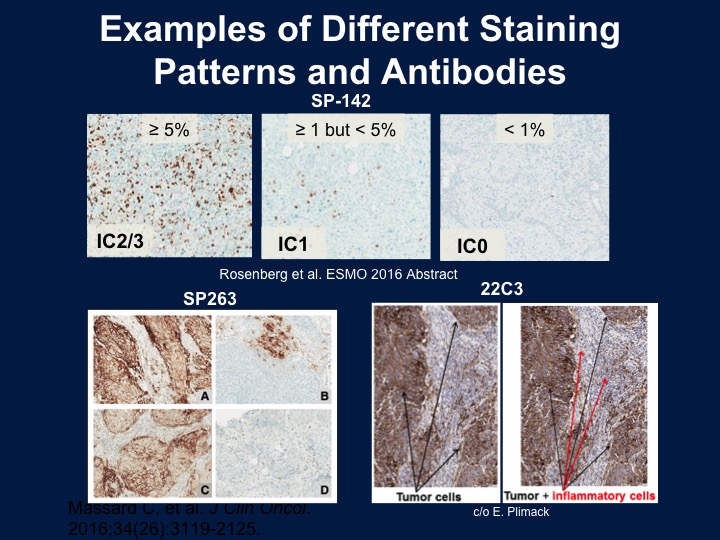
Examples of Different Staining Patterns and Antibodies
And these are different staining patterns that you would tend to see with this. On the left would be a 2/3 from the SP142 is strongly positive again only in the immune cells, and the bottom are either tumor cells plus the immune cells or the tumor cells alone.
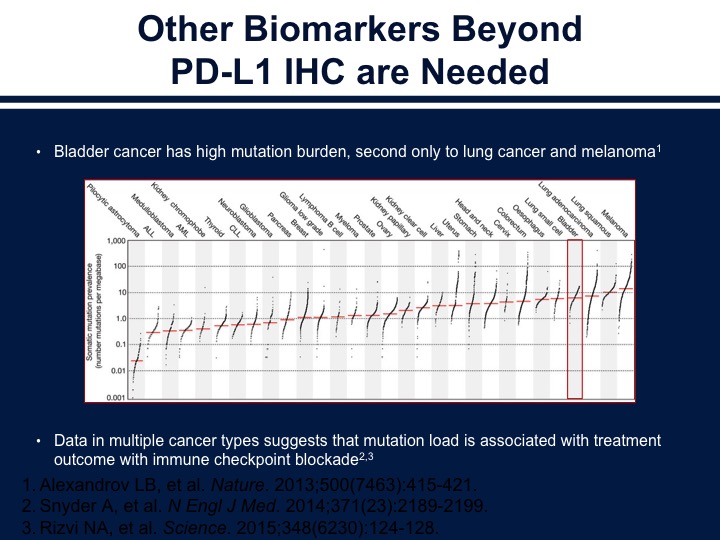
Other Biomarkers Beyond PD-L1 IHC are Needed
Now, we need to look at other biomarkers, and perhaps looking at the tumor mutational load along with immune markers is a way of further refining our prognostic information or predictive information. Bladder cancer is very similar to melanoma and lung cancer in its mutations rate, and this is thought to be related to why it responds to checkpoint inhibition therapy.
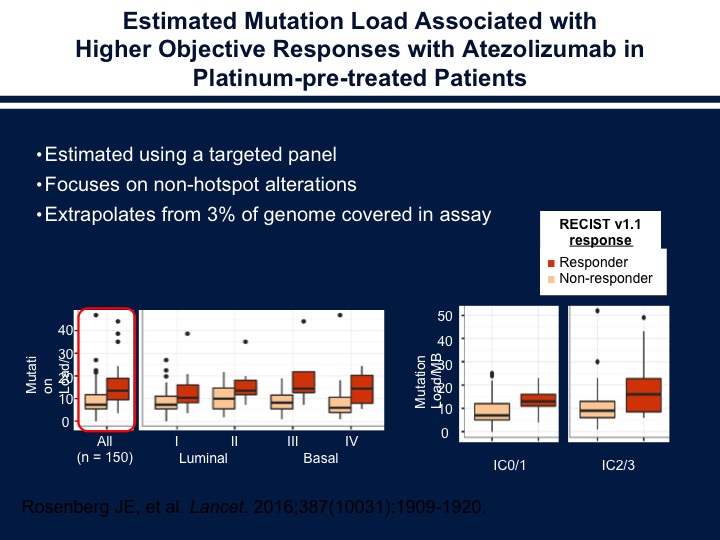
Estimated Mutation Load Associated with Higher Objective Responses with Atezolizumab in Platinum-pre-treated Patients
If we start looking at the different subtypes of bladder cancer and mutational loads we see that there does seem to be a correlation within the individual subgroups of those patients who have high mutational loads versus those patients who have lower mutational loads in terms of how they respond to atezolizumab.
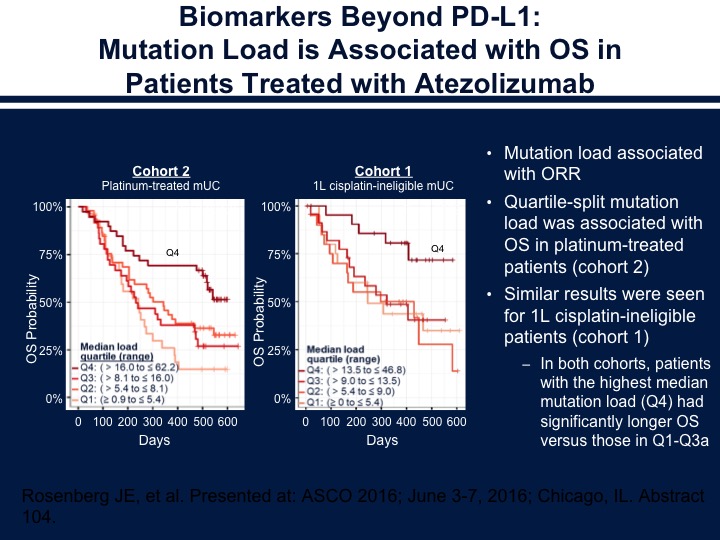
Biomarkers Beyond PD-L1: Mutation Load is Associated with OS in Patients Treated with Atezolizumab
There does seem to be the association with overall survival and the mutational load in those patients who were treated with atezolizumab as well. So the ones that do seem to do the best in this situation are those who were the luminal Type 2. This is what’s felt to be the inflamed phenotype. The basals are what’s thought to be the immune dessert where they don’t express a large number of immune cells and also then the other thing we think about is the fact that you do have the immune cells present and it’s inhibited by the stroma.
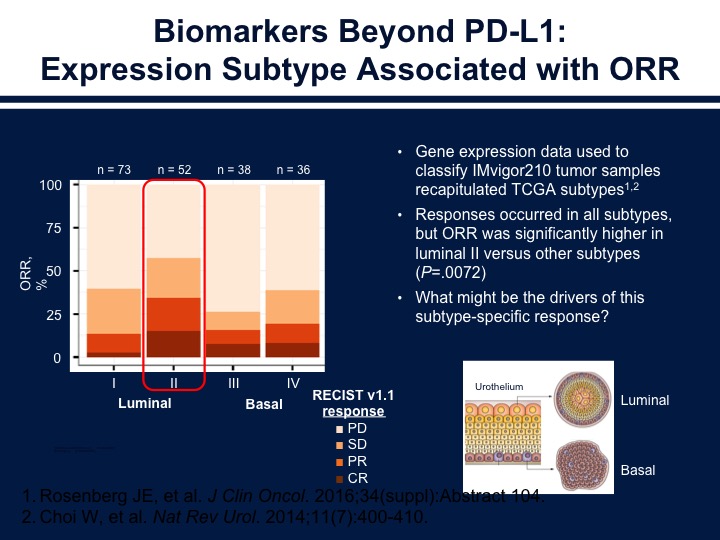
Biomarkers Beyond PD-L1
So again, as we see here the basals have the stromal component that’s inhibiting the immune cells. The luminal type ones are the deserts, I correct myself on that, and the type 2s are the ones that are inflamed. I think that’s again the way we have to start thinking about the disease. A slightly different pattern of response when patients are treated with the nivolumab. The basal ones and the basal twos are the ones that have a higher response rate, and also the interferon gene expression does seem to correlate as we saw with progression, as we saw previously in another talk. I think Lenny went over the BCG in the interest of time. I’ll just skip over the non-muscle invasive disease and go on to some of the other IO combinations that are now under investigation.
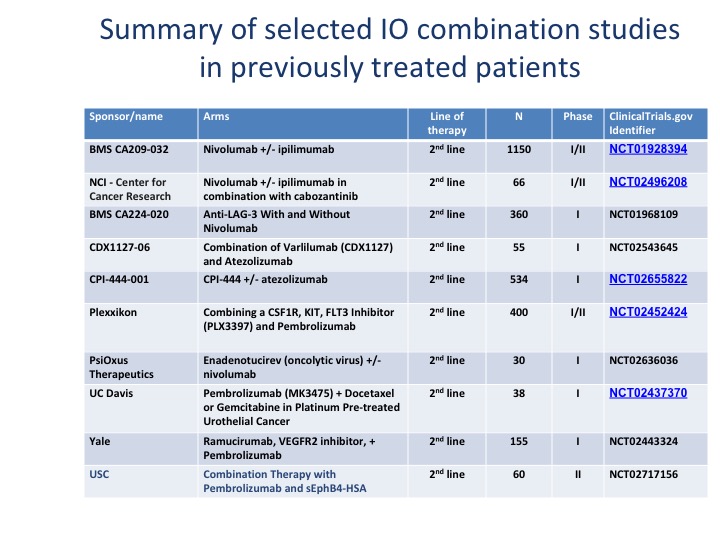
Summary of Selected IO Combination Studies in Previously Treated Patients
We’re looking at a variety of different checkpoint inhibitors. I think the important thing to note about these, especially when you combine ipilimumab with nivolumab or a PD-L1 plus tremelimumab, is your side effects basically double when you add a second checkpoint as well. There are other areas that are being investigated, particularly OX40, which is on the positive end of the immune system. It’s not an inhibitory molecule. There was a trial by Genentech that actually got closed. So there are different portions of the immune system that can be moved forward.
What are the studies evaluating checkpoint inhibition therapy as adjuvant treatment post cystectomy? What is the optimal patient population?
We do have several adjuvant trials that are being evaluated in post-cystectomy patients.
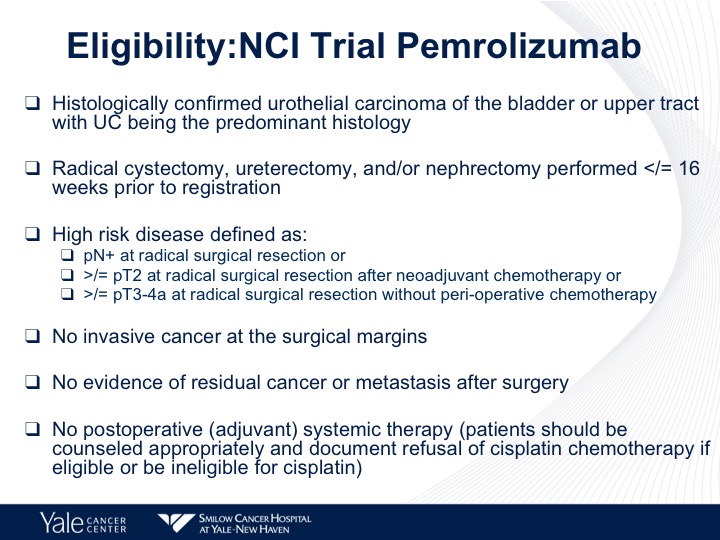
Eligibility: NCI Trial Pembrolizumab
The one I’ll use as an example and there are trials that are being looked at with pembrolizumab as well as nivolumab is the NCI trial, which is now opened. This takes patients who have high-risk disease, who have either node positive disease after surgical resection, greater than pT2 disease after neoadjuvant chemotherapy or greater than pT3 or pT4 disease after surgical resection without perioperative chemotherapy. They can’t have positive margins. They can’t have any evidence of residual disease after surgery.
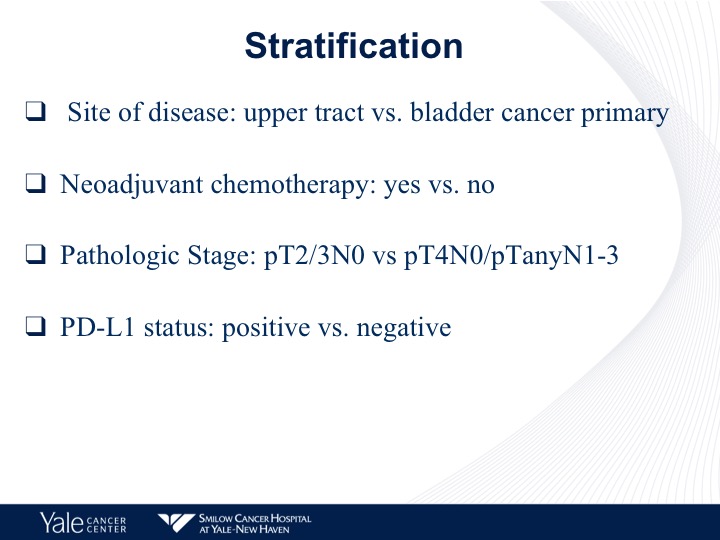
Stratification
And they are stratified by sites of disease, neoadjuvant chemotherapy and pathologic stage as well as PD-L1 status.
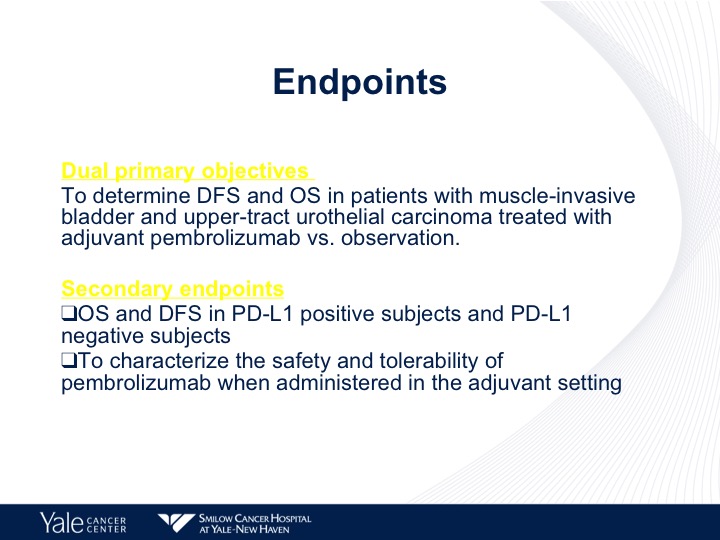
Endpoints
The endpoint is disease-free in overall survival, and then secondary endpoints are disease-free overall survival and PD-L1 status.
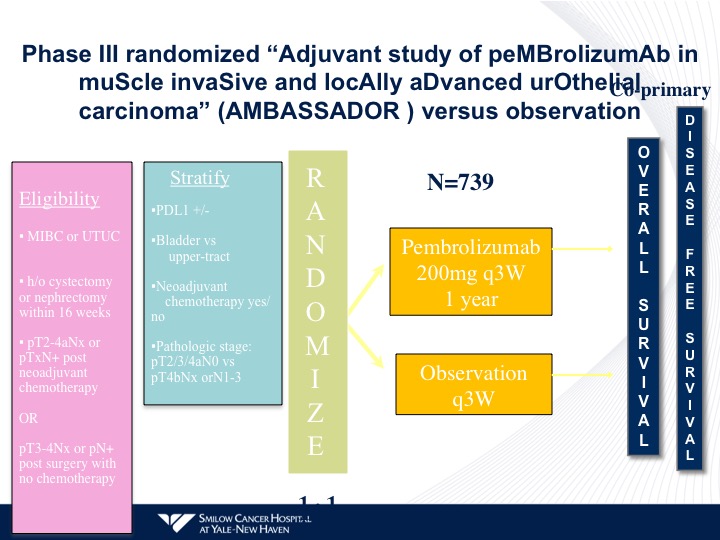
Phase III randomized “Adjuvant study of pembrolizumab in muscle invasive and locally advanced urothelial carcinoma” (AMBASSADOR) versus observation
Very simple randomization. They’re stratified by the criteria I mentioned before. Pembrolizumab versus observation, and pembro is being given for a year. All of the other checkpoint trials are giving one year of either atezolizumab or nivolumab post-therapy. These trials also are including patients with upper tract disease as part of their initial regimens.
Conclusions
So in conclusion, checkpoint inhibition therapy demonstrates significant antitumor activity in cisplatin treated metastatic urothelial carcinoma. Phase I and II trials have confirmed the initial observations of anti PD-L1 and PD-1 therapy in metastatic disease, and I think that we really need a better understanding of the markers. And what we really need are good, consecutive pre and post-treatment studies, and not really rely on archival tissue because, I think that is why we’re having inconsistent answers.
ABOUT THE AUTHOR
Daniel P. Petrylak, MD, is currently Director of Genitourinary Oncology, Professor of Medicine and Urology, Co-Leader of Cancer Signaling Networks, and Co-Director of the Signal Transduction Program at Yale University Cancer Center in New Haven, Connecticut. He is a recognized international leader in the urology field. He earned his MD at Case Western Reserve University School of Medicine in Cleveland Ohio. He then went on to complete his Internal Medicine Residency at Albert Einstein College of Medicine/Jacobi Medical Center in the Bronx, and his fellowship at Memorial Sloan Kettering Cancer Center in New York.
Dr. Petrylak has served as principal investigator (PI) or co-PI on several SWOG clinical trials for genitourinary cancers. Most notably, he served as the PI for a randomized trial that led to the FDA approval of docetaxel in hormone refractory prostate cancer. He also helped to design and served as PI for the SPARC trial, an international registration trial evaluating satraplatin as a second-line therapy for hormone refractory prostate cancer.
Dr. Petrylak served on the program committees for the annual meetings of the American Urological Association from 2003-2011, and for the American Society of Clinical Oncology from 1995-1997 and 2001-2003. He also has served as a committee member for the Devices and Immunologicals section of the FDA. He has published extensively in the New England Journal of Medicine, Journal of Clinical Oncology, Journal of the National Cancer Institute, Cancer Research, and Clinical Cancer Research.