How to cite: Andriole, Gerald L.. “Non-Invasive Molecular Imaging – Fluciclovine” October 3, 2019. Accessed Jan 2025. https://dev.grandroundsinurology.com/non-invasive-molecular-imaging-fluciclovine/
Non-Invasive Molecular Imaging – Fluciclovine – Summary:
Gerald L. Andriole, Jr., MD, discusses the unmet need for precise imaging of biochemical recurrent prostate cancer. He reviews data on imaging agents, especially 18F-fluciclovine PET/CT, ¹¹C-choline PET/CT, and 68Ga-PSMA-11, and deliberates on the impact of imaging-guided treatment changes on patient outcomes.
Abstract:
Many prostate cancer patients experience biochemical recurrence (BCR) after treatment. BCR occurs within 10 years in 20-40% of patients who have radical prostatectomies, and in 30-50% of patients who are treated with radiation.
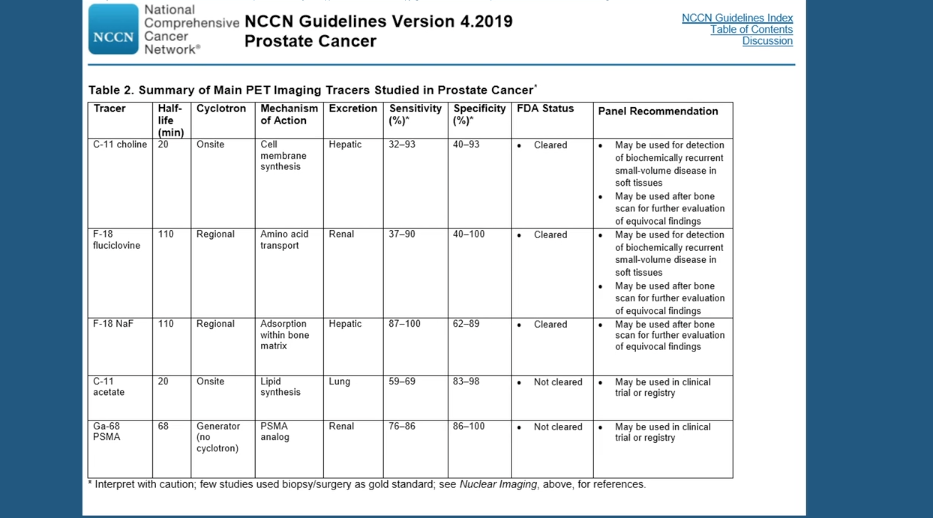
It is often difficult to determine the site of this recurrent disease, and cross-sectional imaging and conventional Tc-99m bone scans are rarely positive in asymptomatic men with PSA < 10 ng/mL. Positron-emission tomography (PET) imaging has shown more promise, though questions remain as to what the most effective PET agent is.
¹¹C-choline PET/CT (choline) and 18F-fluciclovine PET/CT (FACBC) are approved imaging agents for the detection of recurrent disease. Studies show that choline has a pooled detection rate of 62%. However, this agent’s performance varies depending on different factors. Choline has a 36% detection rate for nodal/distant metastases, and only a 25% detection rate for bone metastases. It also has reduced sensitivity and specificity in patients with PSA > 2 ng/mL.
The LOCATE trial found FACBC to have a detection rate of 57%. FACBC has an advantage over choline, however, in its greater sensitivity and superior ability to locate bone metastases. Some researchers have suggested that a third agent, 68Ga-PSMA-11, which is not approved in America, has higher detection rates than FACBC, although some have questioned this study’s validity. Other research indicates that FACBC has an advantage in terms of detecting curable localized disease close to the urinary bladder.
Effective imaging leads to changes in disease management. In the FALCON Trial, ⅔ of patients had a change in management as a result of scanning with FACBC. While It is unclear whether treatment changes affect ultimate outcome, some retrospective studies suggest that metastasis-directed therapy improves survival rates, meaning imaging agents like FACBC may save lives.
About The 4th Global Summit on Precision Diagnosis and Treatment of Prostate Cancer:
The Global Summit on Precision Diagnosis and Treatment of Prostate Cancer is a multi-day, multi-disciplinary forum designated to informing health care stakeholders about topics including in-vitro fluid- and tissue-based molecular diagnostics, novel observation strategies such as active surveillance, and novel therapeutic interventions. Along with this forum’s efforts to form a consensus on the future of prostate diagnostics and precision care, it aims to create an educational and research strategy for its realization. Dr. Andriole presented this lecture during the 4th iteration of this Summit in 2019.
ABOUT THE AUTHOR
Gerald L. Andriole, Jr., MD, is the Robert K. Royce Distinguished Professor and Chief of Urologic Surgery at Barnes-Jewish Hospital, the Siteman Cancer Center, and Washington University School of Medicine in St. Louis, Missouri.
Dr. Andriole received his medical degree from Jefferson Medical College in Philadelphia, Pennsylvania. He trained in surgery at Strong Memorial Hospital and the University of Rochester and completed his Urology Residency at Brigham and Women’s Hospital and Harvard Medical School. Subsequently, he was a Fellow in Urologic Oncology at the National Cancer Institute in Bethesda, Maryland.
Dr. Andriole has over 35 years of consistent contributions in the areas of BPH and prostate cancer screening and prevention research. He has contributed well over 400 peer-reviewed publications and serves on the editorial boards of several prestigious journals. He is Chairman of the Prostate Committee of the National Cancer Institute’s Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial, and PI of the NIDDK Multidisciplinary Approach to Urologic Pelvic Pain (MAPP) and Symptoms of Lower Urinary Tract Dysfunction Research Network (LURN). He was Chairman of the Steering Committee of the REDUCE Prostate Cancer Prevention Trial, as well as PI of both the NIDDK Medical Therapy of Prostatic Symptoms (MTOPS) BPH trial and the NIDDK Complementary and Alternative Medicine for Urinary Symptoms (CAMUS) study. He is a member of the American Urological Association, the American Association for Cancer Research, the American Society of Clinical Oncology, the American Surgical Association, the American Association of Genitourinary Surgeons, and the Clinical Society of Genitourinary Surgeons, among other societies.