Daniel P. Petrylak, MD, presented “PARP in Combination with Standard-of-Care Treatment” during the 3rd Annual International Prostate Cancer Update on January 26, 2019 in Beaver Creek, Colorado.
How to cite: Petrylak, Daniel P. “PARP in Combination with Standard-of-Care Treatment” January 26, 2019. Accessed Dec 2024. https://dev.grandroundsinurology.com/parp-in-combination-with-standard-of-care-treatment/
PARP in Combination with Standard-of-Care Treatment
Daniel P. Petrylak, MD, discusses the potential role of PARP inhibitors in metastatic castration-resistant prostate cancer (mCRPC) treatment. He also discusses the two methods for approaching combination therapy with PARP inhibitors: hypoxia and the anti-androgen blockade.
PARP Inhibitors for mCRPC
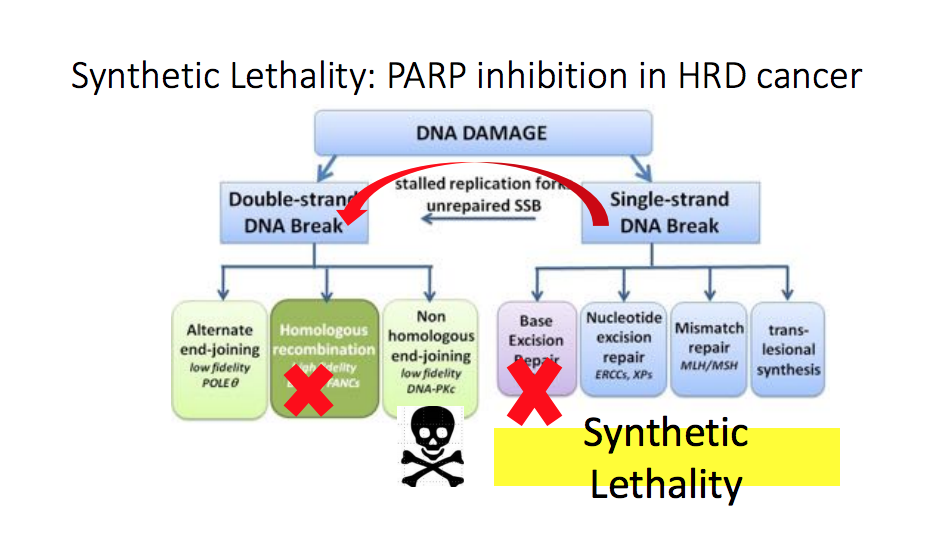
Doctors first used PARP inhibitors, a class of drugs targeting poly ADP ribose polymerase (PARP), as a treatment option in cancers like ovarian cancer. However, results from the TOPARP study suggested that PARP inhibitors are active in prostate cancer as well.
This study looked at 49 patients with metastatic castration resistant prostate cancer (mCRPC) who were docetaxel pretreated. Of the 16 patients who had mutations in the DNA repair pathway, 14 responded to PARP inhibition treatment, whereas only 2 patients out of 33 responded in the group without DNA repair mutations.
Since the TOPARP study, several more studies have begun investigating PARP inhibition in patients with mCRPC. Some PARP inhibitors under investigation as single agent treatments include olaparib, rucaparib, and niraparib.
Combination Therapy
Combination PARP inhibitor therapy may give patients a better chance of responding than monotherapy. The two methods for approaching combination therapy are hypoxia and the anti-androgen blockade. For example, cediranib is an agent that induces hypoxia. Cediranib is an anti-angiogenesis agent that effects VEGFR-1/2 and decreases angiogenesis, vascular permeability, and the survival of new blood cells. Researchers at Yale University are investigating cediranib in combination with olaparib. The belief is that this combination will have a higher response rate (RR) and progression free survival (PFS) than olaparib alone in mCRPC patients.
On the other hand, combining PARP inhibitors with anti-androgens may be a viable combination. Anti-androgens are thought to enhance the blockade of androgen synthesis and increase BRACness. The phase II NCI 9012 study is investigating the combination of abiraterone acetate and veliparib. Overall, in the study so far, there have been no significant differences in PSA decline or PFS. Therefore, this study has not met its primary endpoint. Notably, there has been a higher RR in patients with DNA repair enzyme mutations than those without.
Results of another phase II trial, which compared olaparib in combination with abiraterone to abiraterone monotherapy in patients with previously treated mCRPC, showed about a 5.6 month increase in PFS in the combination arm. However, a difference in overall survival (OS) is not apparent at this point in the study. Consistent with results from other studies, about 15% of patients in this trial had DNA repair mutations. Promisingly, there does seem to be a difference in favor of the combination in patients with DNA repair mutations. Other trials involving checkpoint inhibitors and PARP inhibitors show that there seems to be activity in this combination. For instance, even patients without DNA repair mutations are responding to the combination of pembrolizumab and olaparib.
Conclusions
PARP inhibitors do have activity in patients with aberrant DNA repair pathways. Many combination studies are investigating inducing BRACness to improve efficacy of PARP inhibitors in patients without DNA repair mutations.
About the International Prostate Cancer Update
The International Prostate Cancer Update (IPCU) is an annual, multi-day CME conference focused on prostate cancer treatment updates. The conference’s faculty consists of international experts, and the event caters to urologists, medical oncologists, radiation oncologists, and other healthcare professionals. Topics encompass prostate cancer management, from diagnosis to treating advanced and metastatic disease. Dr. Petrylak presented this lecture during the 29th IPCU in 2019. Please visit this page in order to learn more about future IPCU meetings.
ABOUT THE AUTHOR
Daniel P. Petrylak, MD, is currently Director of Genitourinary Oncology, Professor of Medicine and Urology, Co-Leader of Cancer Signaling Networks, and Co-Director of the Signal Transduction Program at Yale University Cancer Center in New Haven, Connecticut. He is a recognized international leader in the urology field. He earned his MD at Case Western Reserve University School of Medicine in Cleveland Ohio. He then went on to complete his Internal Medicine Residency at Albert Einstein College of Medicine/Jacobi Medical Center in the Bronx, and his fellowship at Memorial Sloan Kettering Cancer Center in New York.
Dr. Petrylak has served as principal investigator (PI) or co-PI on several SWOG clinical trials for genitourinary cancers. Most notably, he served as the PI for a randomized trial that led to the FDA approval of docetaxel in hormone refractory prostate cancer. He also helped to design and served as PI for the SPARC trial, an international registration trial evaluating satraplatin as a second-line therapy for hormone refractory prostate cancer.
Dr. Petrylak served on the program committees for the annual meetings of the American Urological Association from 2003-2011, and for the American Society of Clinical Oncology from 1995-1997 and 2001-2003. He also has served as a committee member for the Devices and Immunologicals section of the FDA. He has published extensively in the New England Journal of Medicine, Journal of Clinical Oncology, Journal of the National Cancer Institute, Cancer Research, and Clinical Cancer Research.