M. Scott Lucia, MD, presented “Pathologist’s Perspective on Focal Therapy” during the 24th Annual Southwest Prostate Cancer Symposium on April 13, 2019 in Scottsdale, Arizona.
How to cite: Lucia, M. Scott. “Pathologist’s Perspective on Focal Therapy” April 13, 2019. Accessed Dec 2024. https://dev.grandroundsinurology.com/pathologists-perspective-on-focal-therapy/
Pathologist’s Perspective on Focal Therapy – Summary:
M. Scott Lucia, MD, defines the ideal candidate for targeted focal therapy of the prostate. He discusses the limitations of transrectal ultrasound- and multiparametric MRI-guided biopsies for determining focal therapy eligibility, as well as evidence regarding template-guided transperitoneal mapping biopsies for improved tumor assessment.
Abstract:
Focal therapy (FT) of the prostate is a treatment option for low-intermediate risk prostate cancer that potentially offers good cancer control with minimal treatment-related morbidities. In addition to clinical features and imaging, the pathologic features seen through biopsy, including grade, location, and tumor extent, are important for determining FT eligibility. Optimal candidates for FT include patients with unilateral, unifocal, low-volume tumors seen on multiparametric magnetic resonance imaging (mpMRI) and those with a Gleason score of less than or equal to 3+4.
Transrectal ultrasound (TRUS)-guided biopsies by themselves are inaccurate for determining the precise location and grade of tumors. Combining systematic TRUS biopsies with mpMRI-fusion biopsies affords improved ability to identify significant prostate cancers. Unfortunately, mpMRI and TRUS biopsies still have limited sensitivity, specificity, and negative predictive value for clinically significant disease, and may underestimate tumor burden.
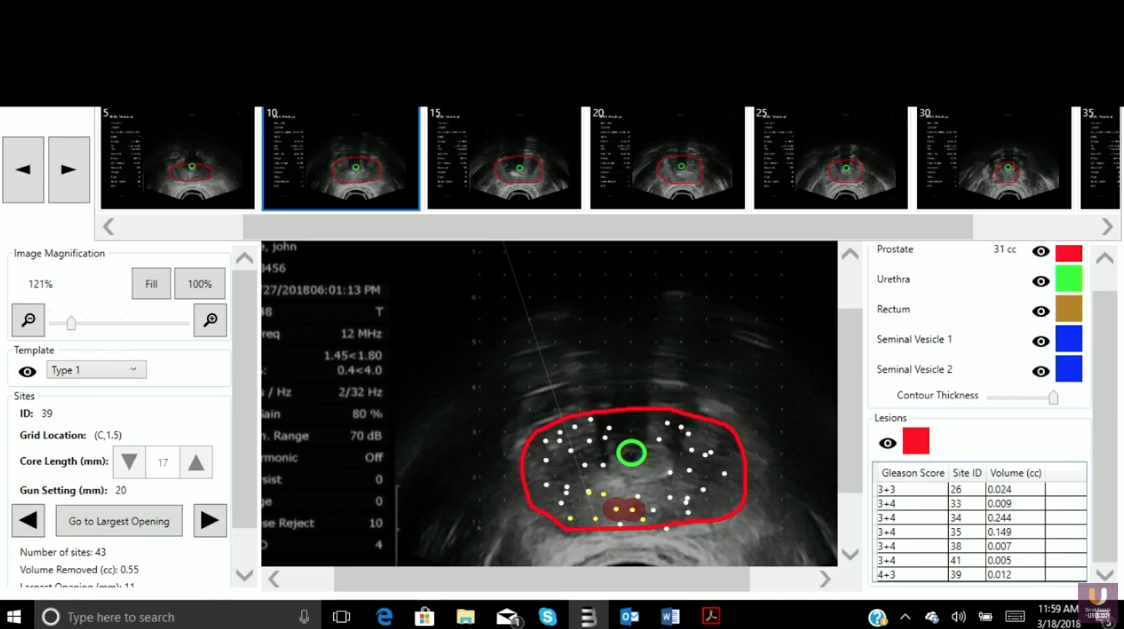
Template-guided transperitoneal mapping biopsies (TTMBs) provide the most accurate assessment of tumor grade, location, and volume. However, TTMBs are technically more difficult and costly to perform.
Early data on TTMB, as well as other studies, have found significant rates of upgrading associated with TTMB when compared with TRUS biopsy and rates of identification of significant bilateral tumor foci undetected by mpMRI+TRUS biopsies.
For post-FT monitoring, mpMRI+TRUS biopsies are recommended at least after 12 months. Long term follow-up of TTMB, the roles of newer 7T MRI technology in determining focal therapy eligibility, as well as the roles of biomarkers in therapeutic monitoring have yet to be determined.
About the Southwest Prostate Cancer Symposium
The Southwest Prostate Cancer Symposium (SPCS) is a multi-day conference that seeks to educate urologists, radiation oncologists, medical oncologists, and other healthcare professionals involved in the treatment of prostate cancer. The topics focus on current technical aspects of diagnosis and treatment of localized and advanced disease, particularly regarding imaging, technology, and training in the related devices. Dr. Lucia presented this lecture during the 24th SPCS in 2019. In 2020, the 25th SPCS will also offer training sessions involving imaging, scanning, and prostate cancer treatment related devices on site. Please visit this page in order to register for future SPCS meetings.
ABOUT THE AUTHOR
M. Scott Lucia, MD, is Professor and Vice Chair of the Department of Pathology and Director of Anatomic Pathology and of the Prostate Diagnostic Laboratory at the University of Colorado Anschutz Medical Campus (UCAMC) School of Medicine. He also serves as the Director of the Prostate Cancer Research Laboratories at UCAMC and of the UCAMC Biorepository Core Facility. Dr. Lucia received his MD from the University of Colorado School of Medicine in 1988. He completed his internship and residency in pathology at the University of Colorado in 1993. He was a research fellow in the Laboratory of Chemoprevention at the National Institutes of Health from 1993 to 1995, before returning to the University of Colorado in 1995.
Dr. Lucia served as the primary pathologist for the Prostate Cancer Prevention Trial (PCPT) and Vitamin E and Selenium Chemoprevention Trial (SELECT), sponsored by the Southwest Oncology Group; the Medical Therapy of Prostate Symptoms (MTOPS) trial, sponsored by the NIDDK; and the Reduction with Dutasteride of Clinical Progression Events in Expectant Management of Prostate Cancer (REDEEM), sponsored by GlaxoSmithKline. He directs the operation of several tissue and serum biorepositories for prostate and prostatic diseases, including those for the PCPT, MTOPS, SELECT, and the University of Colorado Cancer Center Prostate Biorepository. He has authored or co-authored over 180 peer-reviewed articles, reviews, editorials, and book chapters. His primary areas of interest include pathology of prostate cancer and hyperplasia, early detection and prevention of prostate cancer, prostate cancer biomarkers, and mechanisms of carcinogenesis.