
E. David Crawford, MD asked expert Francisco G. La Rosa, MD, FACS about reporting Gleason grades.
Question by E. David Crawford, MD: Dr. La Rosa, there seems to be some variation on how prostate biopsies are reported. My recollection of the Gleason system is that the most predominant pattern is reported. Please explain how a biopsy may be reported as a Gleason eight whereas the tumor may be a Gleason seven (i.e. 3+4).
Answer by Francisco G. La Rosa: This apparent discrepancy may be due to two different scenarios: (1) Since prostate cancers are usually multifocal, TRUS biopsies may be targeting different tumors, one may be 4 + 4 = 8 and another nearby tumor may be a mixture of Gleason grades 3 and 4. The only way to determine that these are different tumors is with examination of a whole mount prostatectomy section. (2) If the tumor is unifocal and TRUS biopsies show differences in Gleason grades, this is due to the heterogeneity of prostate tumors, which usually show areas with only one Gleason grade (i.e. Gleason 4 + 4 = 8) and other areas with a mixture of other grades (i.e. 4+3 or 3+4). This is illustrated in the figure below, which shows one section of a whole mount prostatectomy specimen, in which Gleason grades of the tumor have been outlined with green dots for Gleason grade 3 and purple for Gleason grade 4. Depending on the area of the tumor targeted by the needle biopsy, we may obtain different Gleason grade combinations of a Gleason 3 + 4 = 7 tumor.
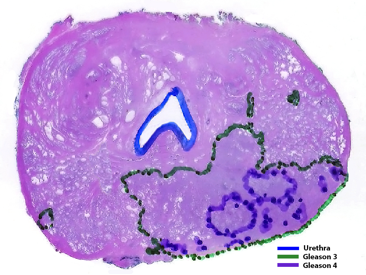
Prostatic adenocarcinoma Gleason grade 3 + 4 = 7 (Gleason grade 4 is ~20%)
Depending on the targeted area, TRUS biopsies will sample different mixed combinations
of Gleason grades. The final Gleason grade will be determined on the whole mount
prostatectomy section.
ABOUT THE AUTHOR
Francisco G. La Rosa, MD, is an Associate Professor in the Department of Pathology at the University of Colorado Anschutz Medical Campus in Denver. He is also a member of the University of Colorado Cancer Center. He is an active clinician, researcher, and teacher, and is board-certified in anatomic and clinical pathology. Additionally, Dr. La Rosa specializes in genitourinary, renal, and heart pathology, and is an expert in pathology informatics. Dr. La Rosa has more than 90 publications in peer-reviewed journals. He is an active member of many national and international organizations, including the College of American Pathologists, the American Urological Association, the Catholic Medical Association, the American Telemedicine Association, Asociación Iberoamericana de Teleslud y Telemedicina, and the Peruvian American Medical Association. As an investigator in genitourinary pathology, he is a member and study coordinator for the Southwest Oncology Group (SWOG) Genitourinary Committee, and a collaborator with the research groups led by Drs. E. David Crawford and Thomas W. Flaig in innovative technology for the diagnosis and treatment of prostate and urinary cancer.







