Peter Black, MD, presented “Should Variant Histology Change Management of Bladder Cancer?” at the 2nd Annual International Bladder Cancer Update on January 27, 2018 in Beaver Creek, Colorado
How to cite: Black, Peter. “Should Variant Histology Change Management of Bladder Cancer?” January 27, 2018. Accessed Dec 2024. https://dev.grandroundsinurology.com/Should-Variant-Histology-Change-Management-of-Bladder-Cancer/
Summary:
Peter Black, MD, fills in for Ashish Kamat, MD, at the 2nd annual IBCU, discussing why and how clinicians should adapt bladder cancer treatment based on variant histologies. Dr. Black identifies sub-types of bladder cancer and the way gene expression and squamous differentiation play a role in risk-stratifying the disease. He explains the proper management of each sub-type, including MD Anderson treatment algorithms for NMIBC and MIBC.
Should Variant Histology Change Management of Bladder Cancer? – Transcript
Click on slide to expand
Should Variant Histology Change Management of Bladder Cancer
We’re going to talk about variant histology in the context of non-muscle and muscle invasive bladder cancer.
Should Variant Histology change Management of NMIBC?
So the primary question is should variant histology alter how we manage patients with non-muscle invasive bladder cancer, and the answer is an emphatic yes. It makes a difference. It’s an important risk factor when we’re looking at patients.
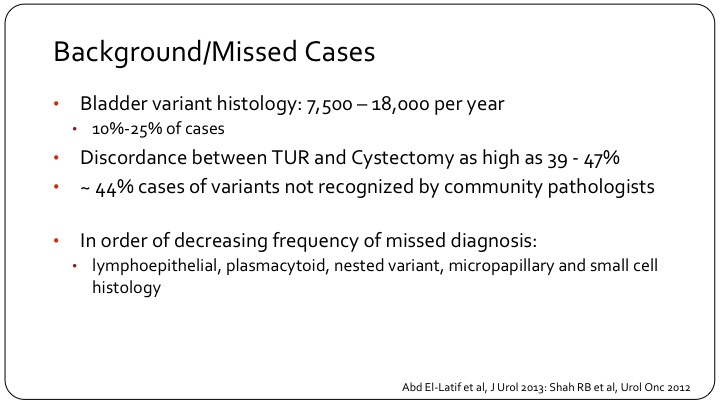
Background/ Missed Cases
To put it in context, although each variant may be rare, up to 10 to 25% of cases will have some variant sub-type in the tumor, whether it’s muscle invasive or non-muscle invasive. We see a great discordance between the TUR and the cystectomy, which is partly due to lack of tissue. It’s partly also due to the fact that TUR may be done in the community setting, and the cystectomy may be seen by a specialized pathologist who is more likely to recognize the variant sub-type. The ones that are most commonly missed are the lymphoepithelial subtypes, the plasmacytoid subtype, which is a bit of a new name that is now being used for signet ring carcinoma of the bladder, also nested variant is a term we’re hearing more and more. Micropapillary and small cell are more established and more readily recognized by the pathologist.
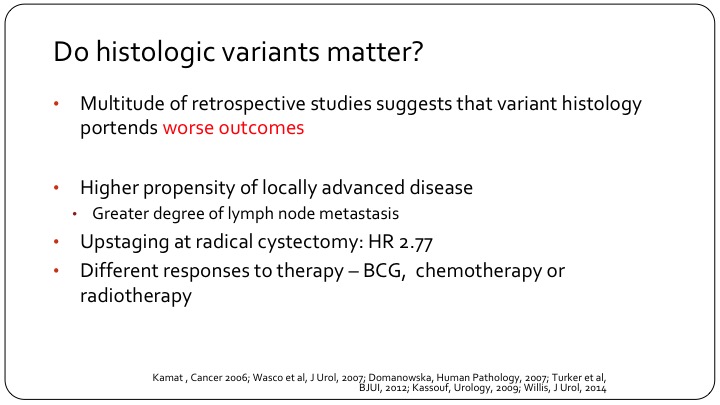
Do histologic variants matter?
There are many studies, most retrospective, single institutional or database studies that indicate that these patients do indeed have a worse outcome. They are more likely to have locally advanced disease, meaning extravesical extension and/or lymph node metastases. We see up-staging more frequently at the time of cystectomy, so especially patients who are clinically node negative but have pathologic nodal involvement and we think, as best we can tell, that they have differential responses to therapy, whether it’s systemic therapy, intravesical therapy, or even radiation therapy.
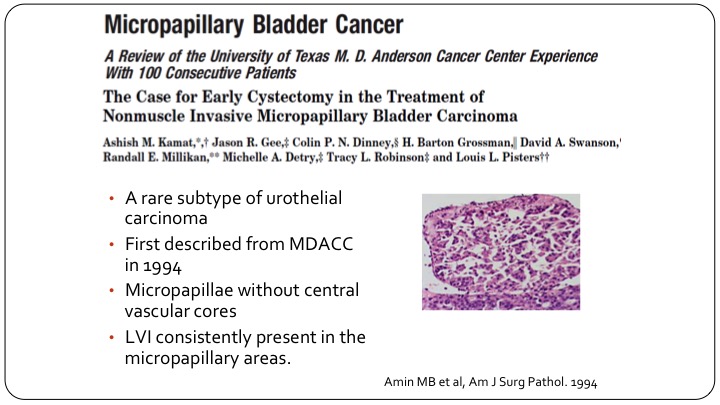
Micropapillary Bladder Cancer
So micropapillary is the one we talk about the most, and it’s the one that is also the best studied. There’s a lot of work on this specifically from the MD Anderson Group. This sub-type was described by the pathologist, Amin, at MD Anderson back in the 1990s, and Ashish and colleagues reported on one of the first experiences with non-muscle invasive micropapillary disease. For the most part, this is T1 disease. It would be extremely rare to see this as Ta or carcinoma in situ. So they are almost all T1s.
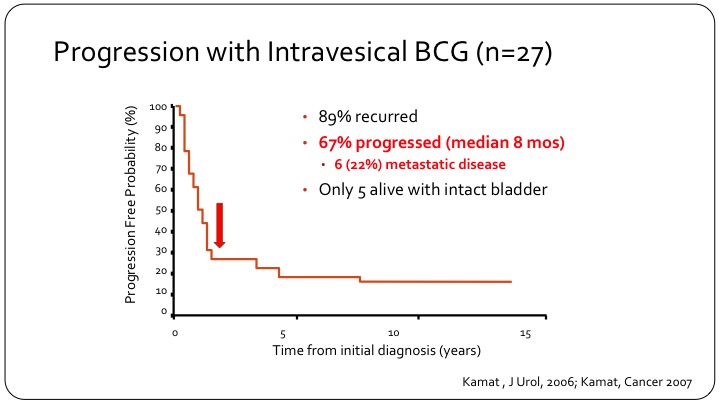
Progression with Intravesical BCG (n=27)
The early experience is highlighted by this one KM curve, which shows that not only did 89% of patients with this disease recur, if you treat with intravesical BCG so these are T1 patients, but 67% so two-thirds of them will progress and they will progress very rapidly indicating that they probably had occult either nodal disease or extravesical disease that was not recognized. In this series of 27 patients, only 5 are alive with an intact bladder at the time of follow-up.
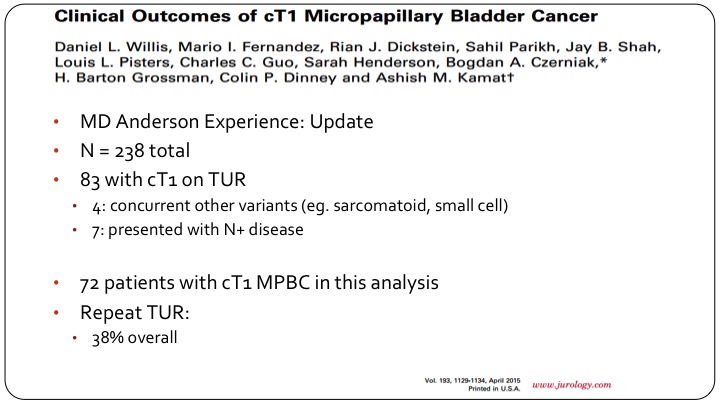
Clinical Outcomes of cT1 Micropapillary Bladder Cancer
Ashish went on and updated this with more patients. They had a total of 238 patients with micropapillary disease, of which 83 were clinically T1 based on TUR. They eliminated a few patients and ended up with 32 for analysis of which 38% had a re-TUR.
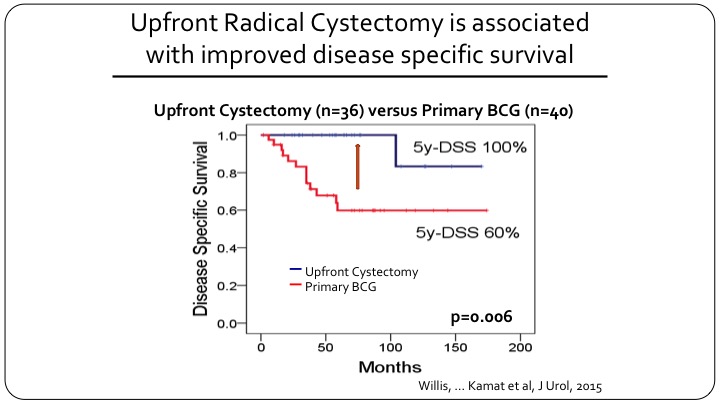
Upfront Radical Cystectomy is Associated with Improved Disease-Specific Survival
They divided the patients into those that had a primary cystectomy versus those who underwent BCG first. It should be 26 cystectomy versus 40 BCG, and you can see the difference in disease specific survival so the patients who had a cystectomy for T1 micropapillary almost all of them survived or did not die of disease, and for primary BCG that number was only 60% at five years.
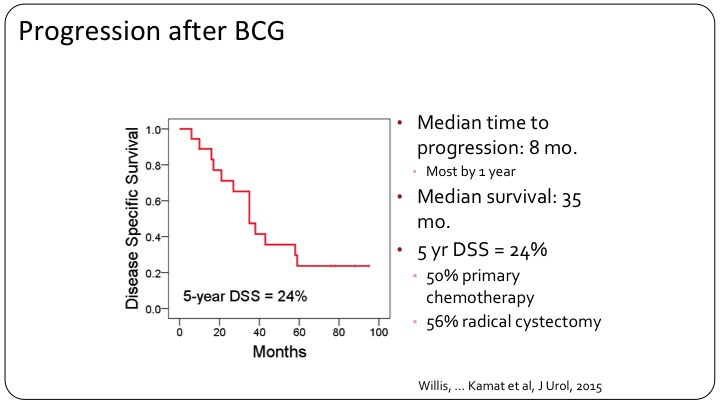
Progression after BCG
If you look at the patients who got BCG, there was a very high rate of progression. Only 24% disease-free at five years. This compares to 50% with primary chemotherapy, so neoadjuvant chemotherapy followed by surgery versus 56% for radical cystectomy alone.
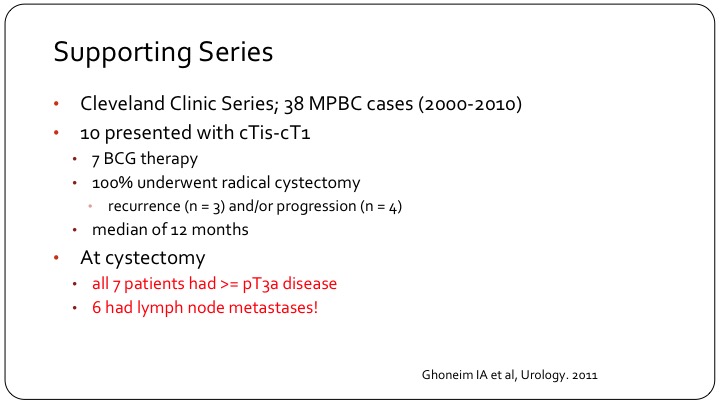
Supporting Series
The Cleveland Clinic also published a very small series, which basically confirms the same thing, very poor outcomes, high rate of occult locally advanced disease. Very small patient numbers. And I think the important counterpoint to this comes from Sloan Kettering.
MSKCC Publication – 2014
This is a paper from Harry Herr’s group essentially that had 36 patients. Some of them received BCG. Some of them underwent primary cystectomy. Both groups did equally well. There are only small differences in disease-specific survival and metastases. These differences did not meet statistical significance, and the conclusion of the authors was that BCG works well in carefully selected patients, and so this remains an open issue with a lot of people pushing for primary cystectomy and others pushing for bladder preservation.
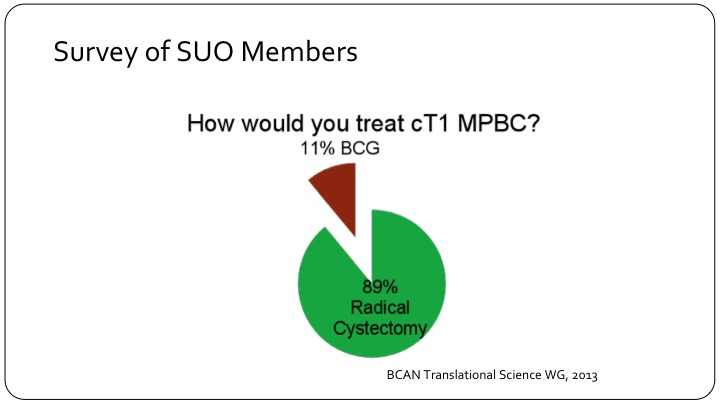
Survey of SUO Members
Based on a working group at the BCAN think tank back in 2013, a survey was sent out to members of the SUO saying how would you treat T1, clinical T1 micropapillary bladder cancer? And there was an overwhelming preference for radical cystectomy, 89%.
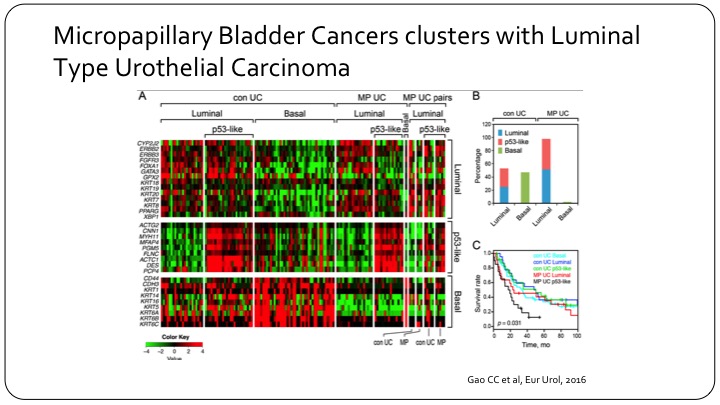
Micropapillary Bladder Cancers Clusters with Luminal Type Urothelial Carcinoma
In the past couple of years, we’ve seen a lot of interesting molecular work on the bladder cancer sub-types. A lot of it has come from MD Anderson. This is one example where they’ve done some gene expression analysis on micropapillary bladder cancer, and really showed that there’s—it provides a biologic background to this morphologic classification. What this slide is trying to highlight is just if you look at conventional urothelial carcinoma you can—as Seth was alluding to you—can divide them into luminal and basal based on gene expression. This, for example would be the basal gene expression. So there’s a lot of red here. But if you take the micropapillary there’s no basal at any point, so they are essentially all luminal, and they can be further sub-divided into just conventional luminal versus p53 like. And this is interesting because basal by itself has a worse outcome than luminal grossly speaking, yet micropapillary has a worse outcome. So micropapillary appears to be a bad-acting group within luminal, and so the molecular underpinnings of that are important.
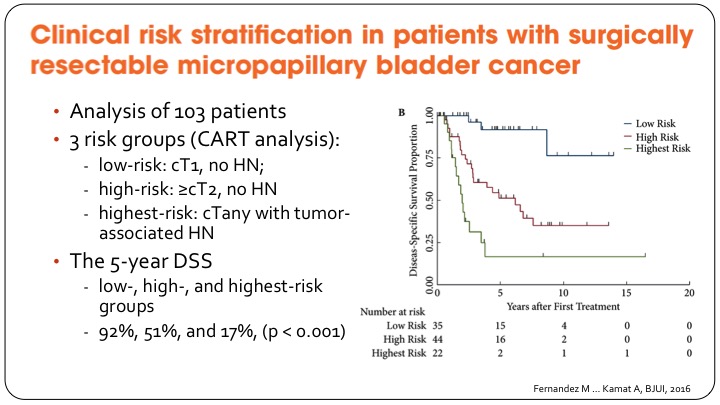
Clinical Risk Stratification in Patients with Surgically Resectable Micropapillary Bladder Cancer
The group at MD Anderson has gone on to provide some clinical risk stratification to help decide what to do with these patients, and they have basically come up with a three-group classification so the lowest risk patients are those with T1 disease and no hydronephrosis, and they have a very good outcome after cystectomy as shown in the KM curve of disease specific survival.
The middle group, which is actually referred to here as high risk they are T2 or greater without hydronephrosis, and then any patient so any T stage but with hydronephrosis they do very poorly I’m going to refer to it as very high risk, so the five-year disease specific survival goes from 92% in the best group to 17% in the highest risk group. It’s based on small numbers but provides some clinical risk stratification.
Small Cell Carcinoma
So I think the other main sub-type that we deal with on a regular basis, if you have a practice that is bladder cancer focused, you will certainly see this regularly, small cell carcinoma. So small cell we clearly separate from conventional urothelial carcinoma without any question, clinically it’s much more likely to metastasize, more likely to metastasize earlier in the disease course. We see some unique metastatic sites, especially brain metastases also more frequent bone metastases. 50% of patients at the time of cystectomy will have metastases although by imaging they were thought to be non-metastatic. So it’s considered a systemic disease. Even a patient with T1 small cell carcinoma, it’s considered systemic disease and it’s generally treated primarily with neoadjuvant chemotherapy.
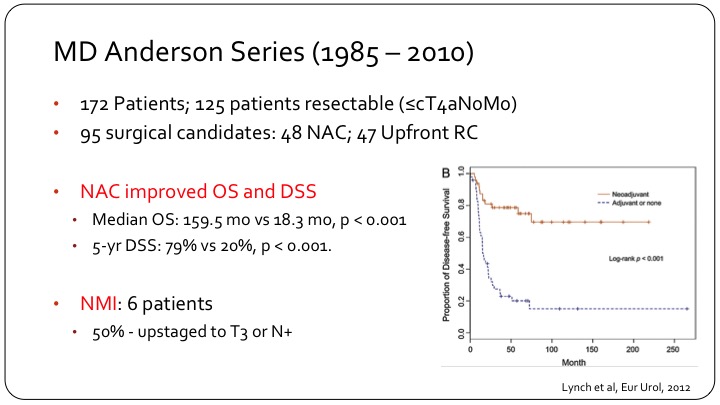
MD Anderson Series (1985 – 2010)
So the MD Anderson Series, they have one of the biggest series in the literature. They had 172 patients of which 125 had resectable disease, organ confined or into prostate or vagina, node negative. 95 actually had surgery of which approximately one-half had adjuvant chemo and one half went for up front cystectomy. And you can see that there is quite a difference in the KM curve on the right of disease free survival, the top line are those who had chemotherapy followed by surgery, and the bottom line are those who went for surgery first with or without adjuvant chemotherapy. So very clearly, these patients need systemic therapy up front, and then local consolidation. Usually the consolidation is surgery, but I think you can make a reasonable argument also for radiation in these patients. As I moved into my environment from a fellowship at MD Anderson, radiation was standard for these patients because that’s what’s done for small cell of the lung, and it’s been very difficult to overcome that bias.
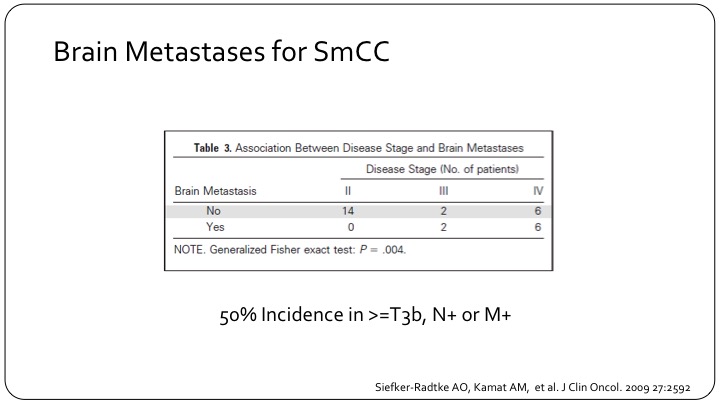
Brain Metastases for SmCC
We mentioned brain metastases, which are common for all small cells of any organ of origin, and these are some numbers that show for patients with more advanced disease. So Stage 3/Stage 4 meaning already metastatic or at least nodally metastatic or T3B disease. That you see brain metastases relatively frequently about half of the patients here. Patients with organ-confined disease, none of them had brain metastases, so we need to be looking for these and it is part of the work up of a patient with small cell variant.
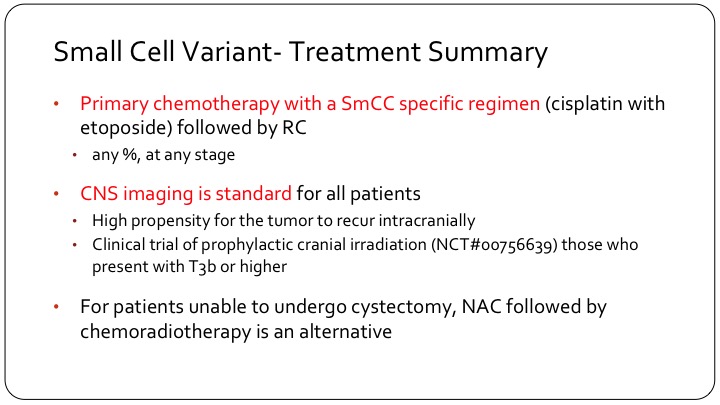
Small Cell Variant—Treatment Summary
So we treat these patients with a different chemotherapy, generally they will get cisplatin/ etoposide, which is borrowed from small cell lung management strategy, and as I said it’s for any stage, even T1 disease, and any proportion of the tumor. It doesn’t have to be greater than 10%, 50%, or anything else. Touched on the imaging so these patients need a brain CT, a head CT, and they generally do not get prophylactic cranial irradiation but the group at MD Anderson is doing a trial on this. They have a 30-patient trial where they—the patients will get 30 Gy of radiation over 15 fractions.
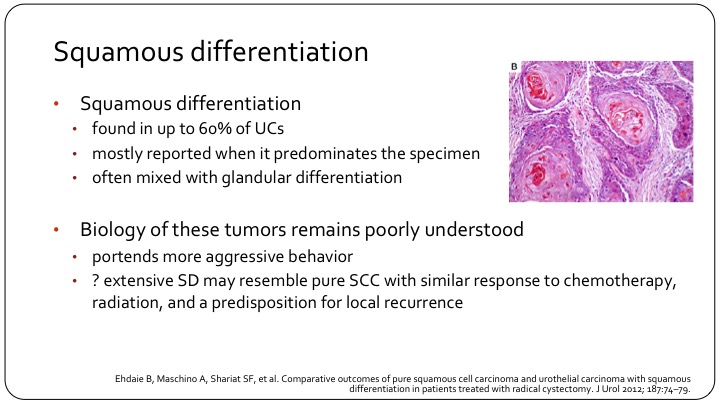
Squamous Differentiation
So another sub-type is squamous differentiation, and this is something that we see very frequently, and the questions is whether we really need to differentiate this from conventional urothelial carcinoma. Up to 60% of bladder cancers will have some degree of squamous differentiation. Patients may also have glandular differentiation, so a component that looks like adenocarcinoma. They may have the glandular without the squamous. There are all sorts of possibilities here. In general, we think that they have a higher risk disease, although it is not completely clear. They are more likely to have extravesical extension, more likely to have nodal involvement. If they have very extensive squamous differentiation, of course, a differential diagnosis from a primary squamous cell carcinoma becomes difficult, and that may have implications for therapy, especially with a choice of neoadjuvant chemotherapy.
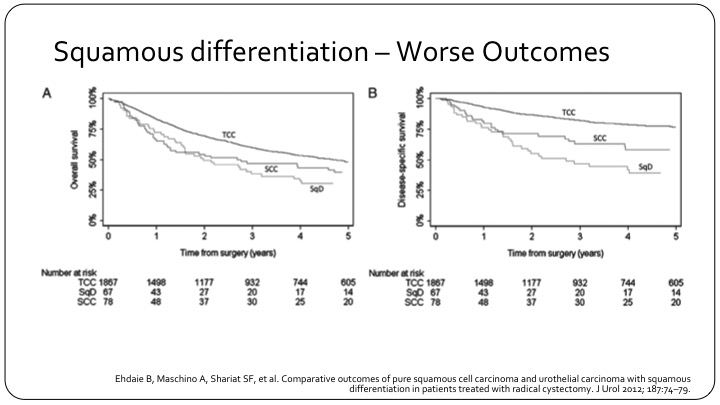
Squamous Differentiation – Worse Outcomes
So this is an example of a large series with over 1900 patients looking at how squamous differentiation affects outcome. On the left is overall survival. On the right is disease specific survival, and you see the top line in each graph is conventional urothelial carcinoma. The bottom line is actually squamous differentiation, and in the middle are the true squamous cell carcinomas.
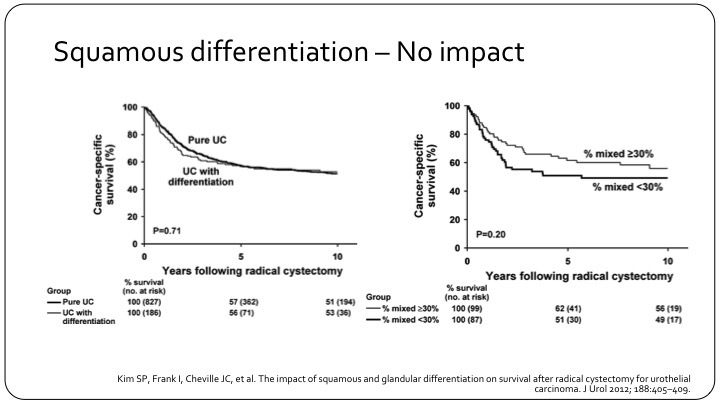
Squamous Differentiation – No Impact
Yet, this is another example of pure versus differentiation that doesn’t really show much of a difference. This is a Mayo Clinic experience, also a good number of patients. So it’s not 100% clear how much it impacts outcome as a prognostic factor.
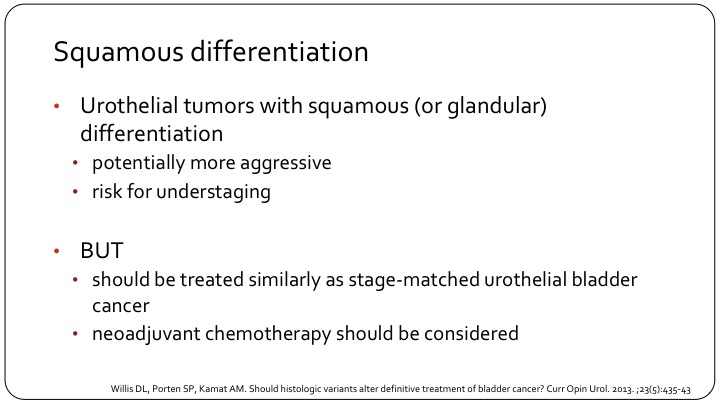
Squamous Differentiation
In general, we should say they should be treated the same, so if it’s non-muscle invasive disease, there is no reason not to give BCG as you would give a patient BCG for if there’s no squamous differentiation. In the muscle massive context, neoadjuvant chemotherapy should still be considered. I hear a lot the statement, whether it’s residents, fellows, other urologists, that since there’s a squamous—a component of squamous differentiation, and we know that squamous cell doesn’t respond well to cisplatin-based chemotherapy, the patient should not get neoadjuvant chemotherapy, and I think that is an error that we need to clarify. There’s some evidence that tumors with squamous differentiation are, in fact, basal and as Seth alluded to, the basal tumors respond best to chemotherapy. So, if anything, you could say that maybe these patients should be prioritized for neoadjuvant chemotherapy. But that also is a bit of a strong statement to make.
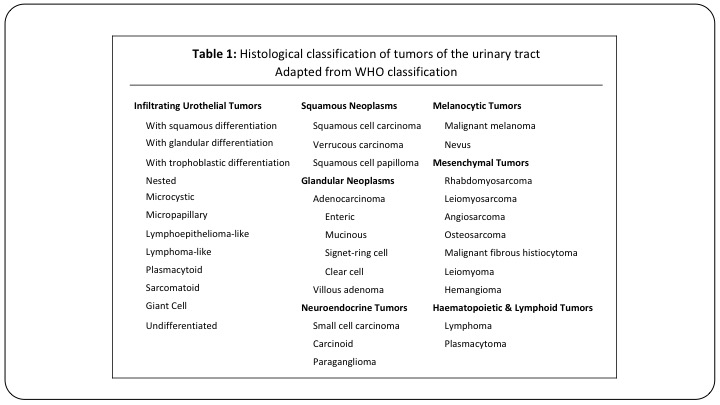
Table 1
Here is just a list of all of the different subtypes that we ultimately need to be familiar with, and a lot of these we will see never or once or twice in a career. But they are important to detect.
Non-urothelial Bladder Cancer and Rare Variant Histologies
So these are a few of the important papers that Ashish has published. Again, Ashish made the slides, I didn’t, but he has published a lot on how variant histology should impact management.
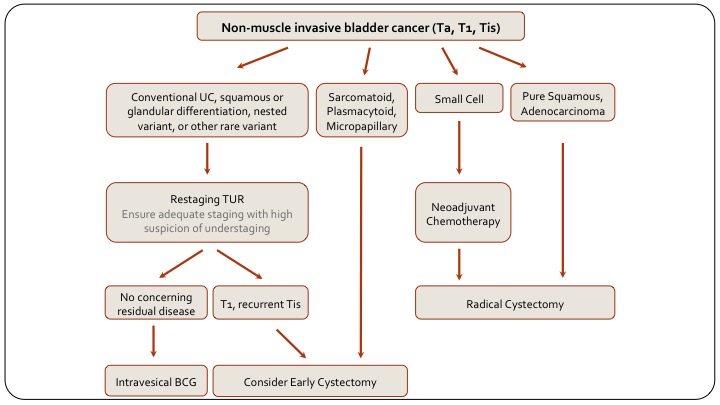
Non-muscle Invasive Bladder Cancer
Just to summarize with the last two slides, this is a slide on how to manage non-muscle invasive disease based on variant histology, and you can see on the left side are your conventional urothelial carcinoma patients and it includes those with squamous or glandular differentiation because that doesn’t really differentiate how we’re going to treat them. And so non-muscle-invasive disease we would typically re-TUR, then decide on intravesical BCG or early cystectomy, as we otherwise would anyway. If they have sarcomatoid plasmacytoid or micropapillary disease, they should go straight top cystectomy. So it’s non-muscle invasive disease with these variants. Small cell, as we said we give them chemotherapy, pure squamous or pure adenocarcinoma in this context are really like these ones, they should also have primary cystectomy.
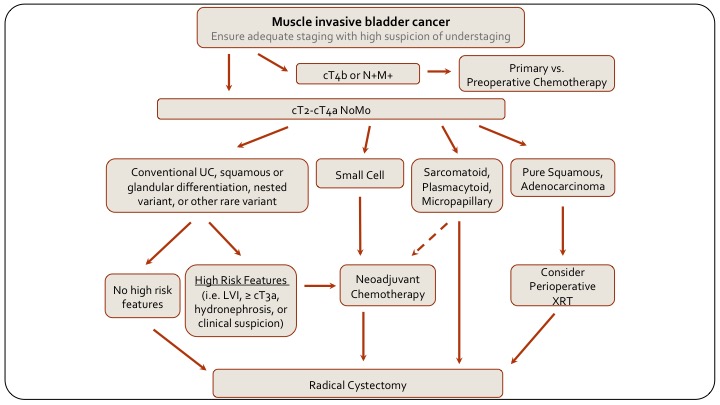
Muscle Invasive Bladder Cancer
And then, if we look at muscle invasive disease and how the variants play in, first of all if they are not, you know, if they have public wall invasion, they are T4B or rectal involvement, or node positive metastatic, obviously, if they have chemotherapy as their primary treatment, but if they are resectable, then again, if there is squamous differentiation or glandular differentiation, they are treated the same as conventional, this again is an MD Anderson algorithm. A lot of us would say neoadjuvant chemotherapy regardless, but the alternative is to risk-stratify based on risk features and only give neoadjuvant chemo to those with higher risk features and go straight to cystectomy in the absence of risk features. If they have small cell, they get chemo followed by cystectomy, or radiation I would say, and if they have sarcomatoid, plasmacytoid or micropapillary, again they typically would go for radical cystectomy just because we don’t know how neoadjuvant chemotherapy works in these patients and we don’t want to take the risk of delaying radical cystectomy for potentially futile chemotherapy. That also is a little bit controversial. I know from my training at MD Anderson, that even the different bladder cancer specialists at MD Anderson did a little bit differently.
Again, sarcomatoid for example is a basal tumor and perhaps there is an advantage to chemotherapy, but we don’t know. And then of course off to the side, squamous and adenocarcinoma, typically they go straight to cystectomy. It’s always interesting to consider radiation because it’s done in other organ sites, but there is no real evidence to support its use in bladder cancer. That is the end. Thank you.
Thank You
Question from the Audience:
Could you clear up for me please the basal and the luminal differentiation in these tumors? Is that an anatomical location? Is that a characteristic of the cell itself?
Dr. Black’s Answer:
So that’s a very good question. It’s based on RNA expression, so it’s a completely molecular thing right now, and the idea is if you look at the molecular pattern of these tumors they either resemble luminal cells of the normal urothelium or they resemble basal cells, so luminal meaning more differentiated and basal meaning more stem like, so their gene expression patterns resemble those patterns of normal urothelium. You can differentiate also with immunohistochemistry. So there are specific proteins you can look at that will roughly divide it along the same lines. But, for the most part, when we’re talking about it, we’re talking about RNA expression.
ABOUT THE AUTHOR
Dr. Peter Black is a urologic oncologist at Vancouver General Hospital, a research scientist at the Vancouver Prostate Centre, and a Professor in the Department of Urologic Sciences at the University of British Columbia (UBC). He received his undergraduate degree from UBC and his medical degree from Johannes Gutenberg University in Mainz, Germany. He completed his urologic training at the University of Washington in Seattle and a Fellowship in Urologic Oncology at MD Anderson Cancer Center in Houston, Texas. He has a clinical subspecialty interest in bladder cancer. He maintains a grant-funded translational research program in urothelial carcinoma and is an active cancer clinical trialist in this field.