Neal D. Shore, MD, presented “The Gold Standard for Bladder Cancer Detection” during the 3rd Annual International Bladder Cancer Update on January 23, 2019 in Beaver Creek, Colorado.
How to cite: Shore, Neal D. “The Gold Standard for Bladder Cancer Detection” January 23, 2019. Accessed Dec 2024. https://dev.grandroundsinurology.com/the-gold-standard-for-bladder-cancer-detection/
The Gold Standard for Bladder Cancer Detection- Summary:
Neal D. Shore, MD, discusses the benefits of using blue light cystoscopy (BLC) in adjunct with white light cystoscopy (WLC) in the diagnosis and treatment of bladder cancers. He then reviews the body of evidence evaluating the accuracy of BLC in detecting significant tumors and briefly compares BLC and narrow band imaging.
Abstract:
While white light cystoscopy (WLC) is the gold standard for bladder cancer detection, there is room for improvement with this technique. Due to the chronic nature of the disease, bladder cancer is very expensive to manage. Surveillance and recurrence costs account for the majority of this economic burden. Improving imaging techniques can help alleviate this issue, as well as improve patient care.
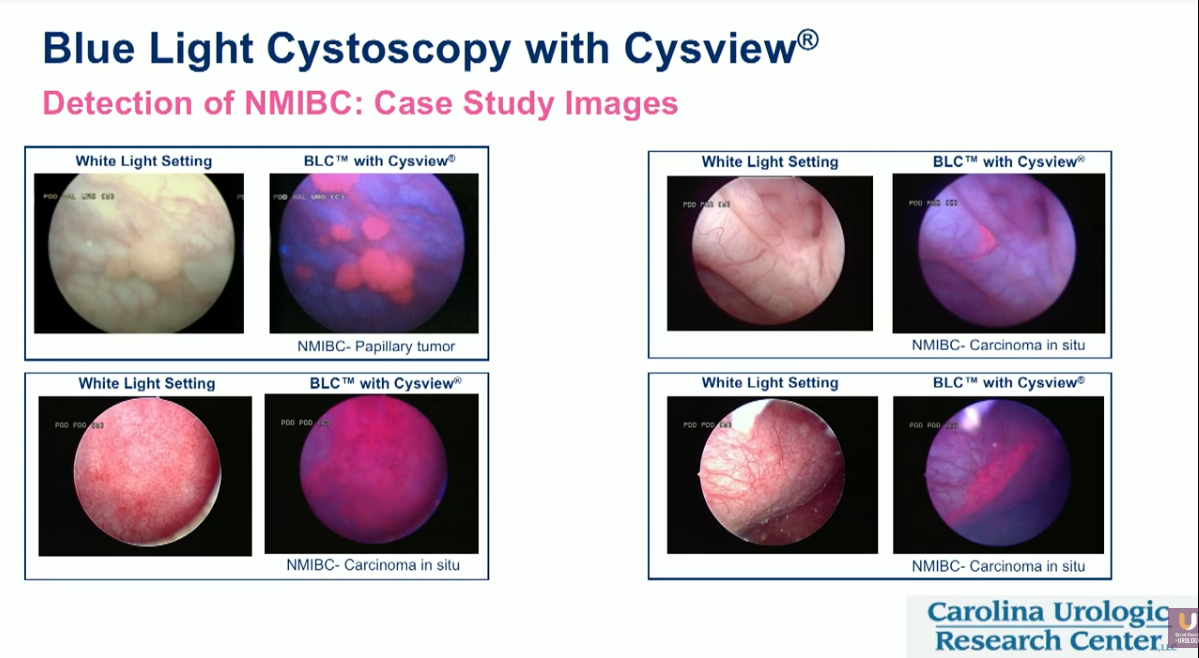
Blue light cystoscopy (BLC) can augment the use of WLC to improve both the detection and resection of bladder cancers. BLC is an optical imaging agent and supplement to WLC that involves the insertion of a photosensitizer into the bladder. Under blue light illumination, abnormal cells fluoresce red, enabling the detection of cancer cells not visible in white light settings.
Notably, BLC can specifically produce defined visualizations of carcinoma in situ (CIS), papillary tumors, small lesions, and, conceivably, large tumors around the margins of the bladder.
The body of clinical evidence evaluating BLC shows a pronounced difference between visualizations made solely with WLC, and those made with WLC paired with BLC. In a phase III, comparative, multicenter study, BLC significantly improved detection of reccurent bladder cancer in 20.6% of patients, and of CIS in 34.6% of patients. In another prospective, randomized study, BLC detected Ta/T1 tumors in 16.4% of patients and CIS in 46% of patients that WLC did not.
BLC is the standard of care throughout most of Europe. Comparatively, the mainstream urologic community in the United States does not use BLC as widely. This is because of reimbursement issues and costs associated with the purchase of needed equipment. In the future, additional related studies are necessary to investigate BLC in the context of disease progression and patient survival.
About the International Bladder Cancer Update
The International Bladder Cancer Update (IBCU) is an annual one-day CME conference focused on bladder cancer treatment updates. IBCU takes place during its sister conference, the International Prostate Cancer Update (IPCU). The conference’s faculty consists of international experts, and the event caters to urologists, urologic oncologists, and other healthcare professionals. In addition to didactic lectures, IBCU features interactive discussions, a panel roundtable, debates, and case presentations. Dr. Shore presented this lecture during the 3rd IBCU in 2019. Please visit this page in order to learn more about future IBCU meetings.
ABOUT THE AUTHOR
Neal D. Shore, MD, FACS, is the Medical Director for the Carolina Urologic Research Center. He practices with Atlantic Urology Clinics in Myrtle Beach, South Carolina.
Dr. Shore has conducted more than 350 clinical trials, focusing mainly on genitourinary oncology, and serves on the executive boards of the Society of Urologic Oncology and the Bladder Cancer Advocacy Network. He is Past President of the Large Urology Group Practice Association. He is a founder for both CUSP Clinical Trials Consortium and DASHKO, a national urology practice data registry. He serves as the National Urology Research Director for 21st Century Oncology. He has served on the AUA Male Health Committee and the AUA Data Committee, the SITC Task Force for Prostate Cancer, the Bladder Cancer Advocacy Think Tank, and the Editorial Boards of Review in Urology, Urology Times, Chemotherapy Advisor, OncLive, PLOS ONE, Urology Practice, and World Journal of Urology. He serves as Editor of Everyday Urology-Oncology. Dr. Shore has written more than 200 peer-reviewed publications and numerous book chapters. He performs peer review for Lancet Oncology, New England Journal of Medicine, European Urology, the Journal of Urology, Urology, BJUI, PCPD, and numerous other high-impact scientific journals.
A graduate of Duke University and Duke University Medical School, Dr. Shore completed a 6-month clinical research fellowship in Pretoria, South Africa, and then completed his General Surgery/Urology training at New York Hospital Cornell Medical Center and at Memorial Sloan-Kettering Cancer Center in New York City. He is a Fellow of the American College of Surgeons.