Keywords: prostate cancer, antigen, CTLA-4, immunotherapy, metastatic, prostatectomy, PSA, Sipuleucel-T, T-cell, immunotherapy for prostate cancer
How to cite: Concepcion, Raoul S. “What’s New in Immunotherapy?” January 26, 2017. Accessed Jan 2025. https://dev.grandroundsinurology.com/whats-new-immunotherapy
Summary:
Dr. Raoul S. Concepcion discusses the shift in urology practices toward using immunotherapy for prostate cancer and bladder cancer, and the rise of checkpoint inhibitors. He cites trials of sipuleucel-T and PROSTVAC-Tricom.
Edited Transcript
Immunotherapy is the newest approach for treating and preventing prostate and bladder cancer.
To explore this topic, I will move the focus away from individual drugs. I feel it is more important to discuss checkpoint inhibitors, CTLA-4, PD-1, PD-L1, and costimulatory molecules when it comes to developing a full understanding of immunotherapy. In order to understand how these drugs work, we need to go back to immunology 101. A couple of years ago, immunology could be taught in a two-week course. Now, it would take two years to teach the subject.
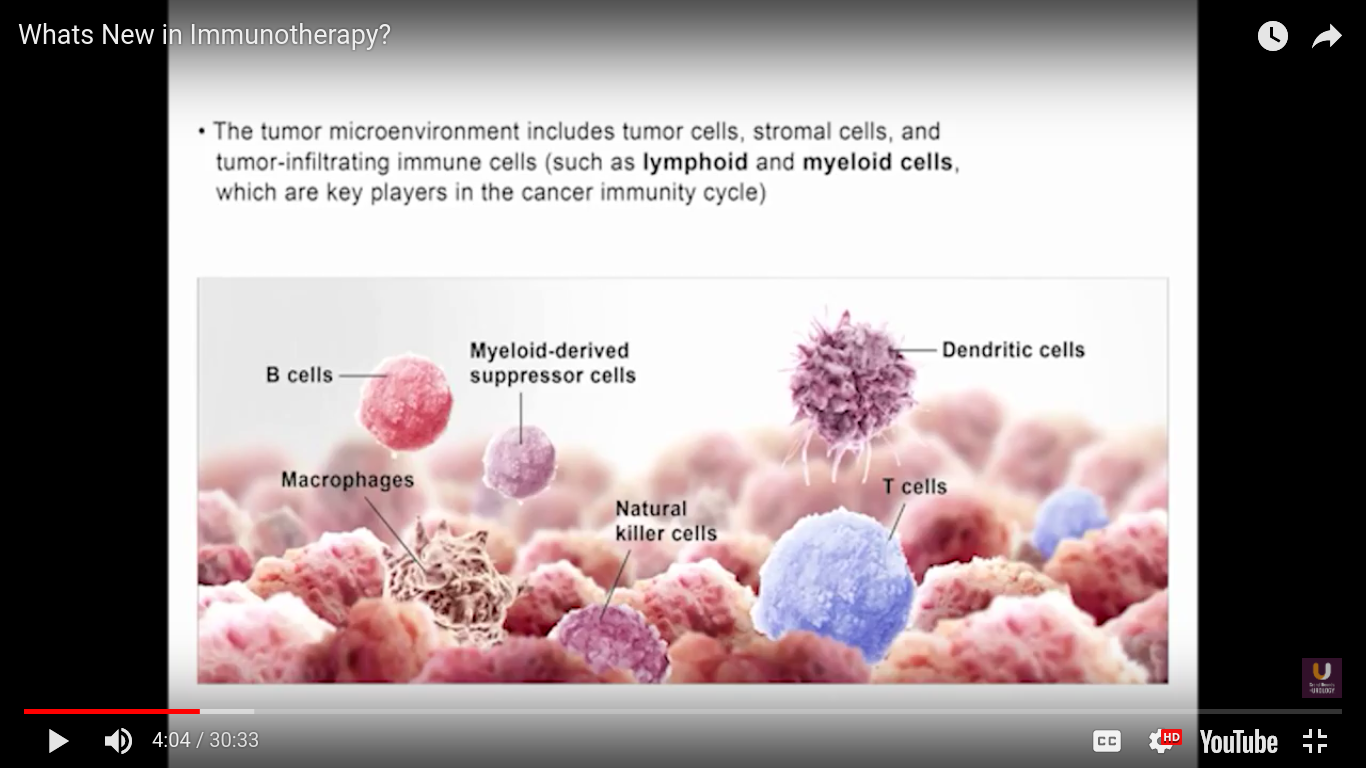
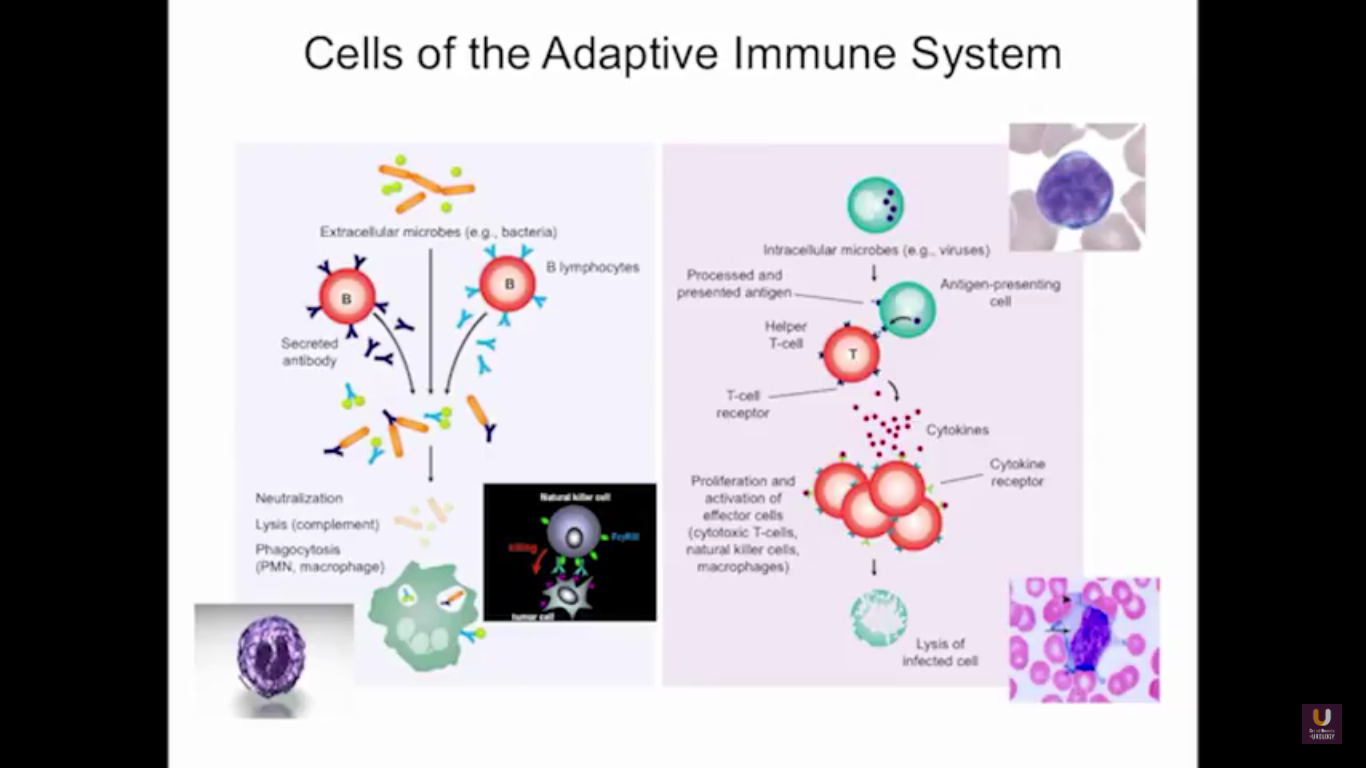
In the category of lymphocytes, T cells and B cells are all antigen specific. Then, natural killers, or NK cells, are cytotoxic lymphocytes. They recognize and kill stress or malignant cells independent of antigen activity. Next, there are androgen presenting cells, or myeloid cells, that are important for T-cell mediated response.
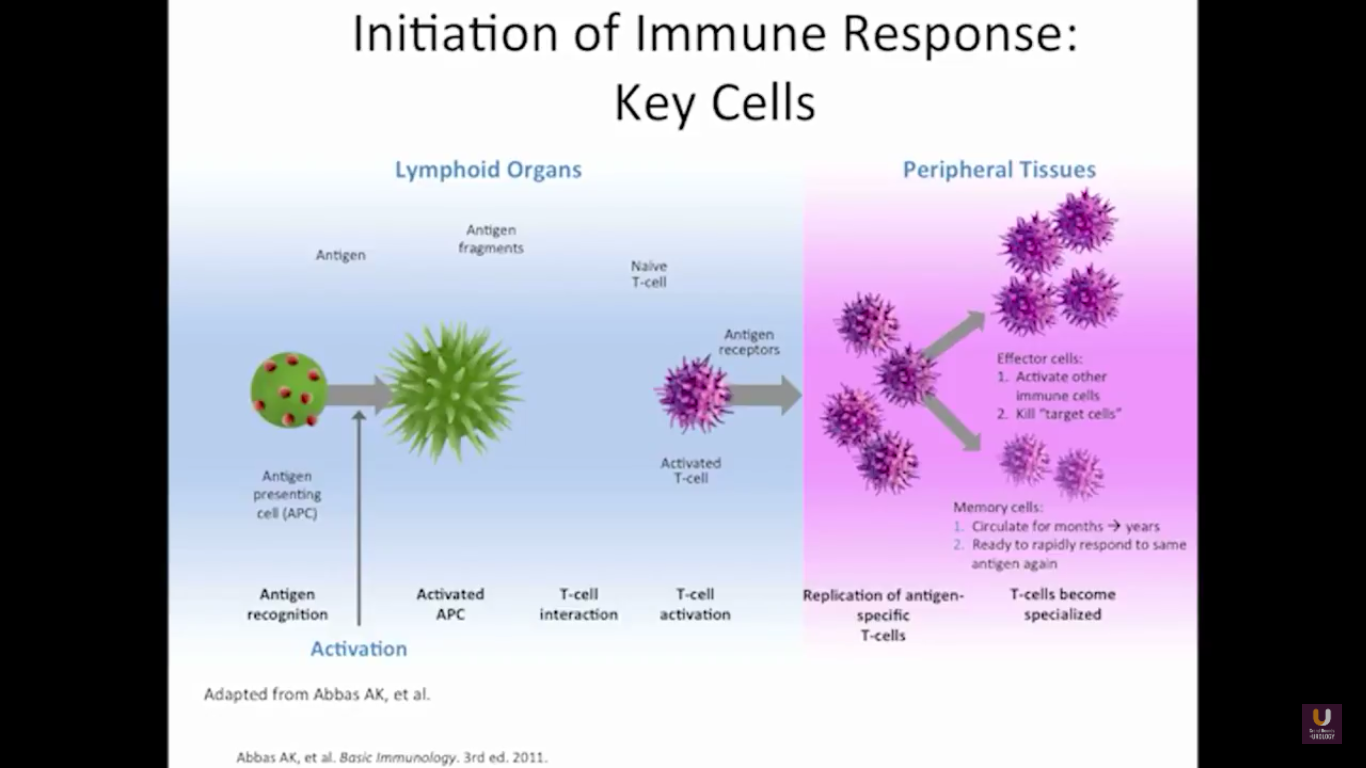

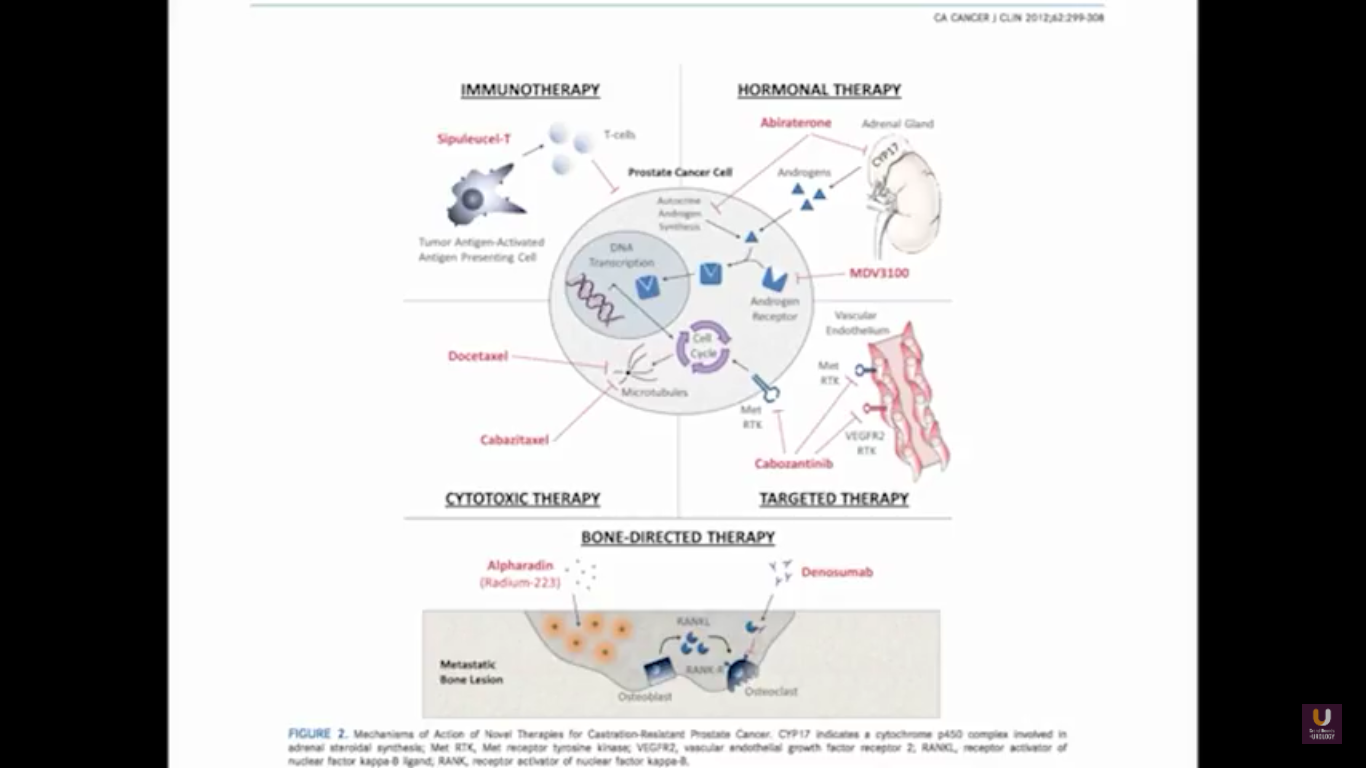
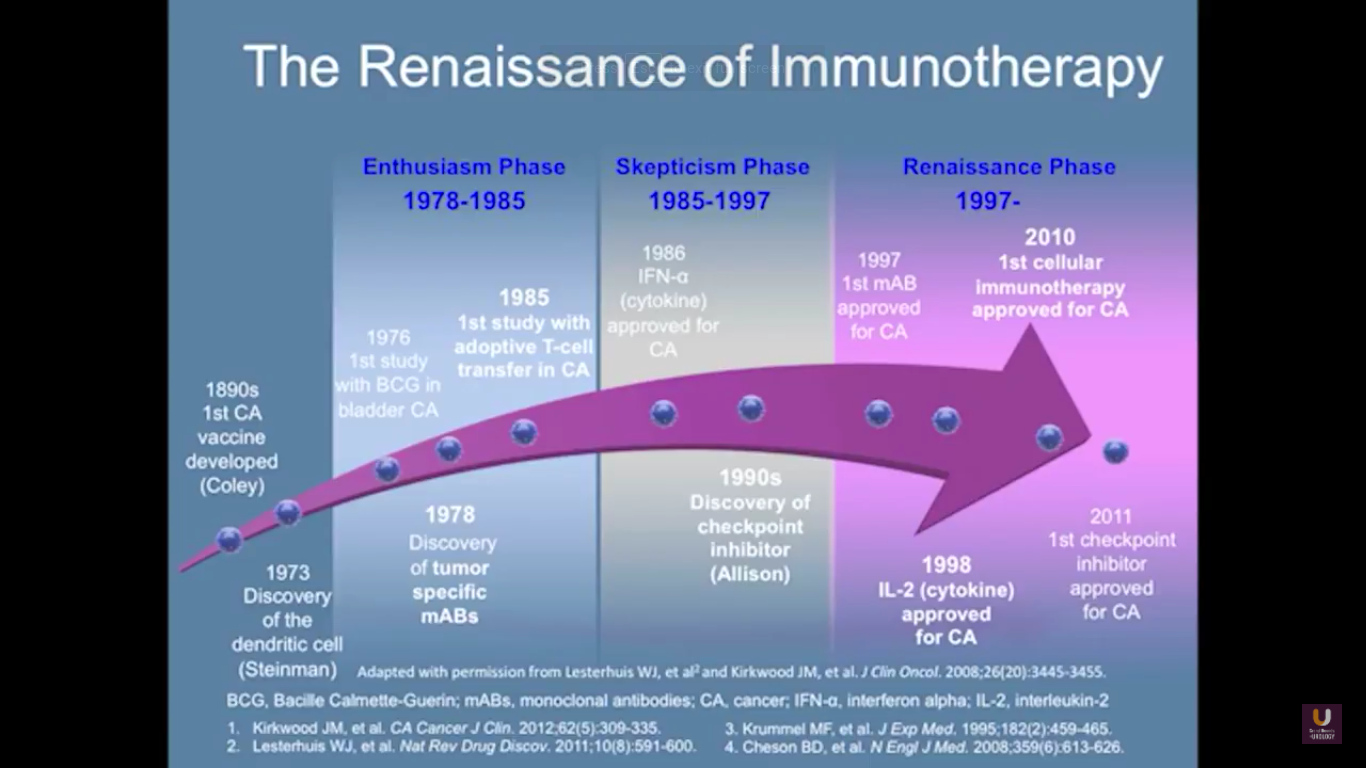
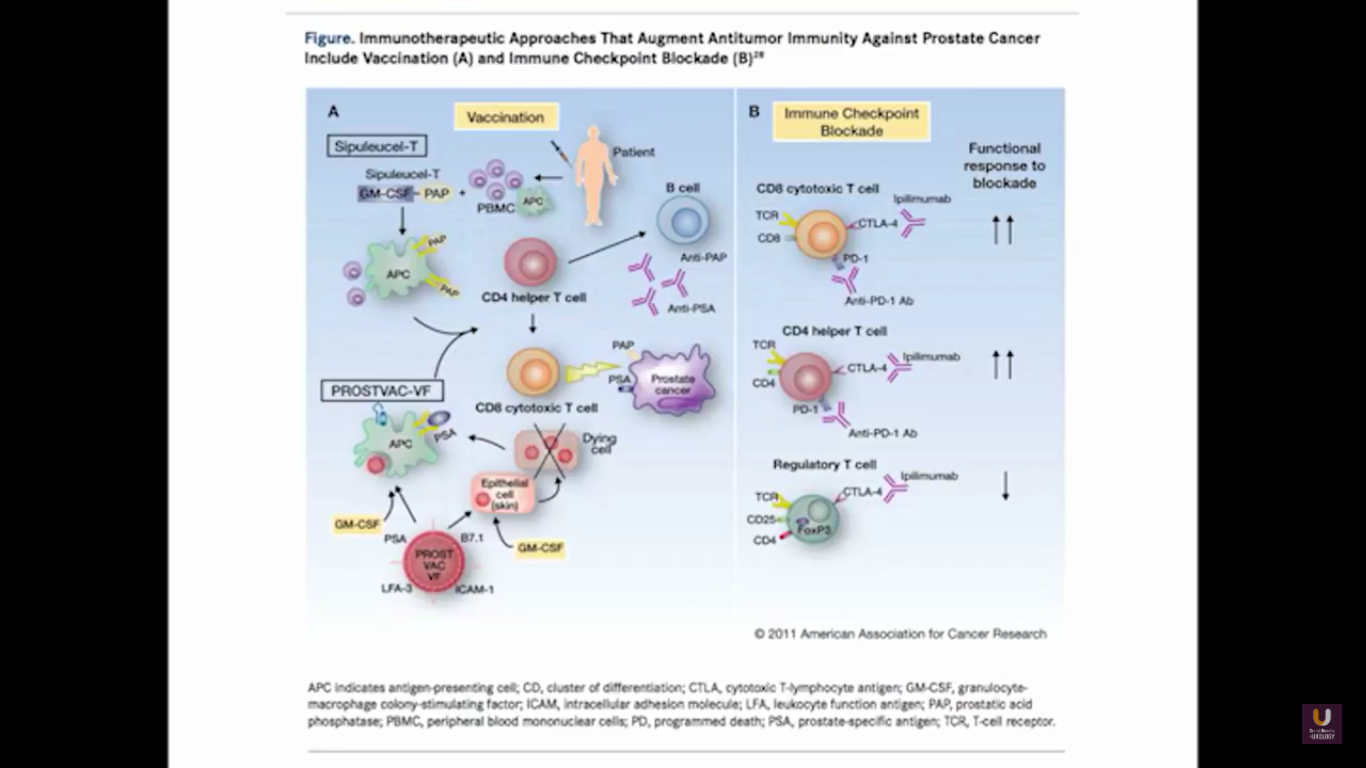
In terms of immunotherapy, over a dozen different immunotherapy agents have been approved since 1986. Most of these agents were approved after 2010. The currently approved agents target over ten different cancer types. Keep in mind this data is mildly outdated. These numbers are expanding rapidly. However, the only approved therapeutic vaccine for prostate cancer is in metastatic castration-resistant disease. It’s called sipuleucel-T, and it was approved in April of 2010.
That’s why we have all these great M0 trials as we move forward, especially with early hormonal agents. Some companies are trying to move some therapeutic vaccines into this M0 space. For now, this is a real problem, because they remain in either clinical trial or observation. But, I think with some newer imaging agents, including FACBC, PSMA, choline, we might see more evidence of metastatic disease. This opens up our play book, if you will, to many newer drugs.
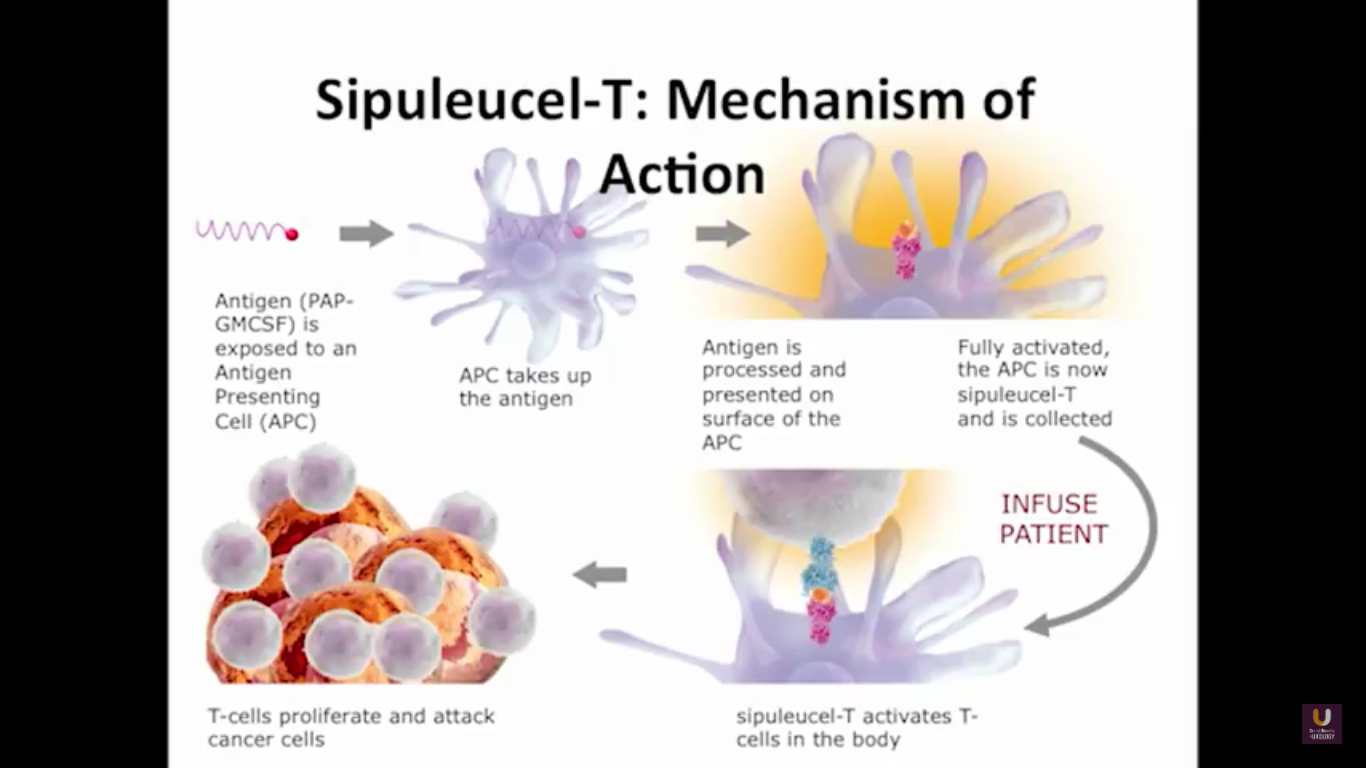
We send the patients for an apheresis. We pull out about 5% of their antigen presenting cells.
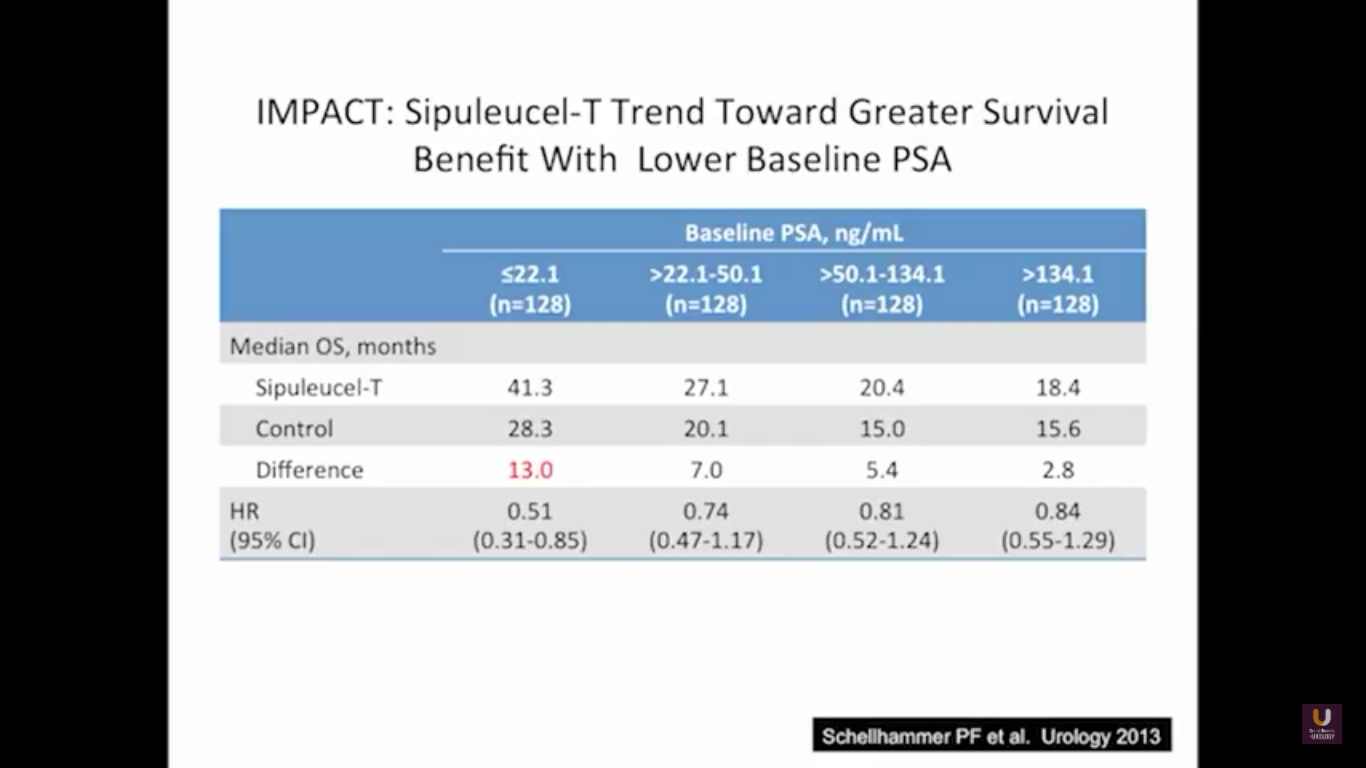
The problem with this trial is that there was not a PSA effect. The PSAs did not go down after treatment, but the patients lived longer. This study showed that patients with lower PSAs, or lower volume of disease, have a longer survival benefit. While the survival benefit held for all four, the most responsive group were people with lower PSAs. Remember that a PSA is an androgen driven event. It is a protein that’s a function of transcription and translation that’s driven by testosterone binding to the androgen receptor. So if we take out testosterone, simply, the cells are making less PSA. Think of this way. Think of two patients with three things in common: they’ve had a radical prostatectomy, they have PSAs of 3, and they have prostate cancer. What they don’t have in common is the first patient has never been on hormone, he’s hormone naïve, and the second patients is on androgen deprivation therapy. What if I asked you the simple question, “Who has more disease? Is it the first guy who is hormone naïve, or is it the second guy on hormones? Do they have the same amount of disease? Do you not know?” The answer has got to be, for the most part, guy number two. His testosterone levels are already low and he’s making less PSA.
This makes perfect sense. This forms the basis of why we advocate to the urology world, and to everybody, that if you’ve got a patient on androgen deprivation therapy and their PSAs are going up, albeit small values, you better start looking for disease. This highlights that if you start these patients early on therapy, they will live longer.
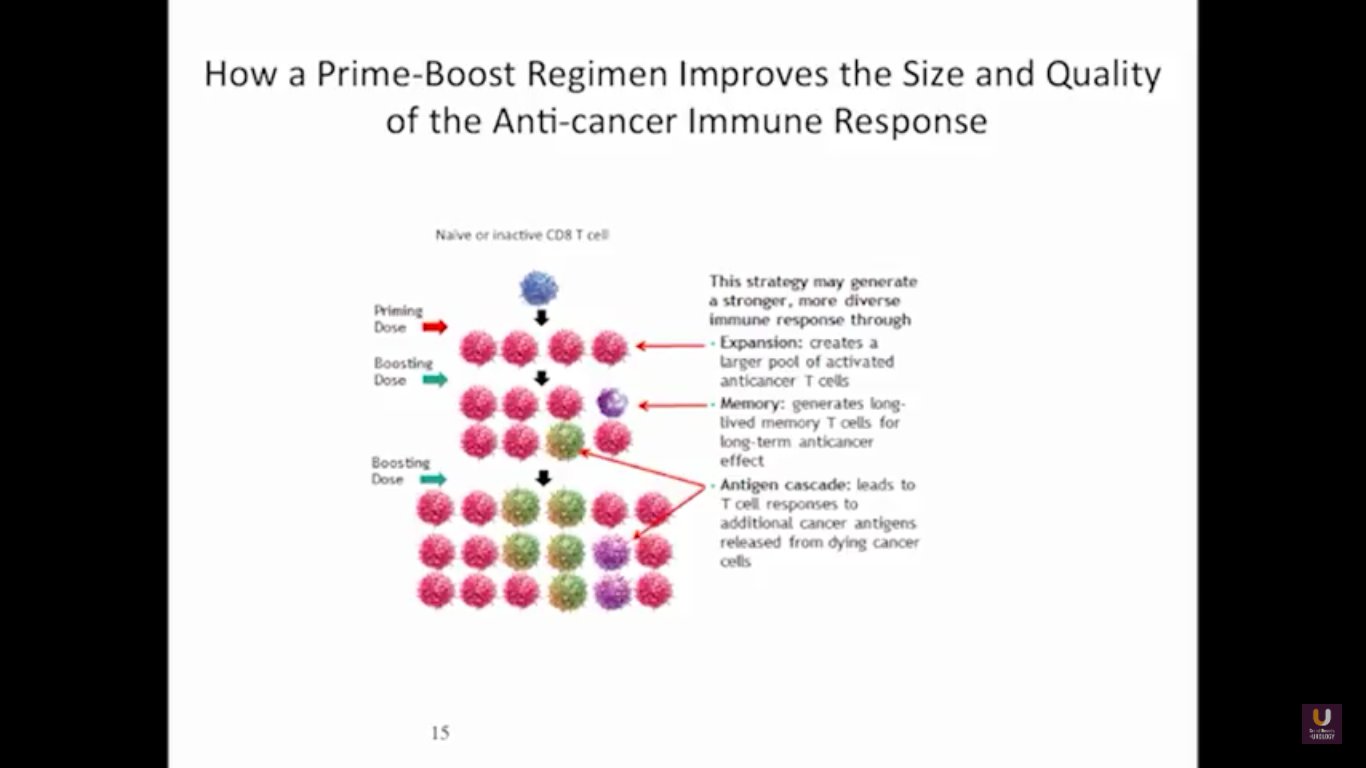
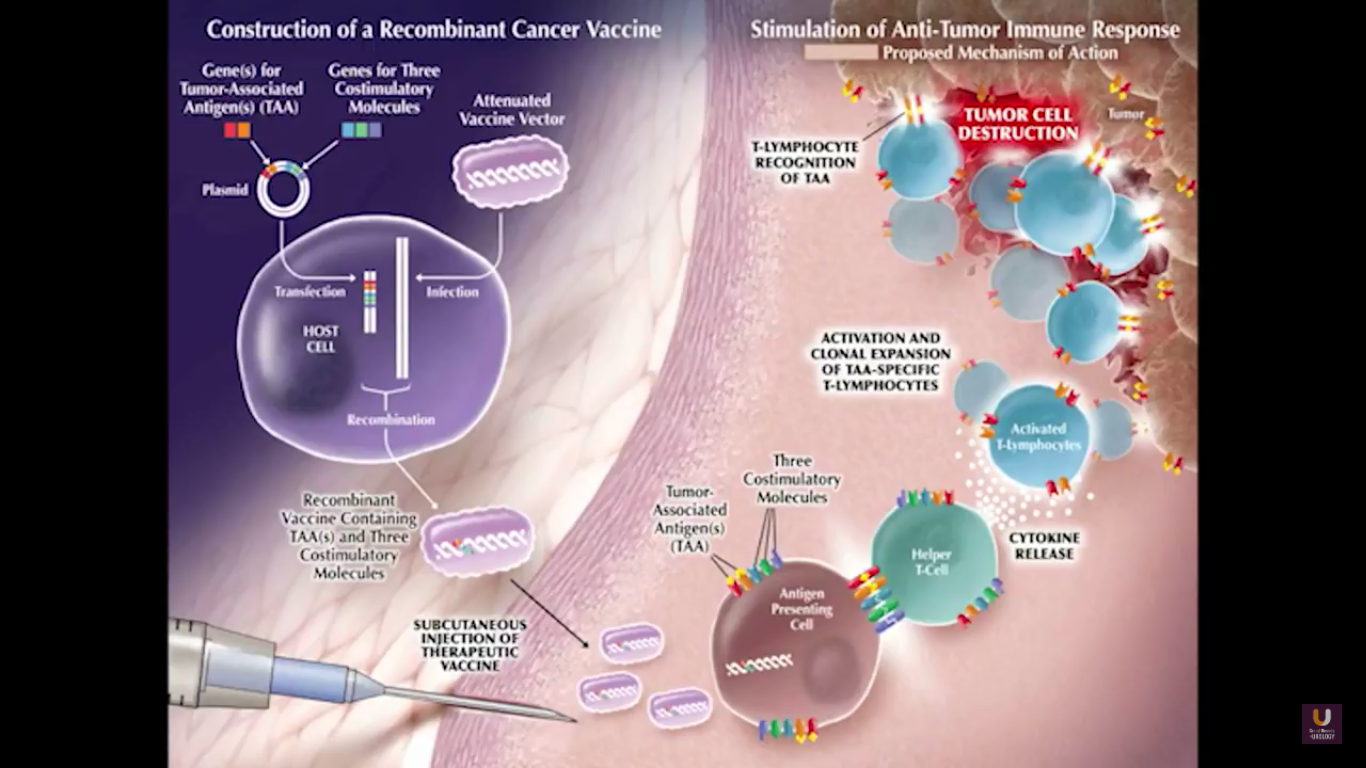
Now, Schellhammer’s study was not powered, but it is pretty significant. If you understand the nuances, especially in the control group of IMPACT, it becomes even more meaningful. Again, I encourage you, be looking for these patients if they’re on ADT when they have small bumps in their PSA. Remember that with sipuleucel-T, we’re actually driving it off the epitope of prostatic acid phosphatase. We’re only targeting one antigen. We know prostate cancer cells express multiple different membrane antigens. Once you get cell kill, then you get release of these antigens, then you get antigen spread. Even though you’re only targeting one particular antigen, in this case PAP, you still get spread and uptake of other antigens which can result in an enhanced immune response. By looking at various expressions of increased antigen spread, you can see it is associated with an increased overall survival if it was PAP only, two secondary antigens, or three secondary antigens. This concept of antigen spread, even though sipuleucel-T only targets one antigen, once you get cell kill, you also get broader antigen spread.
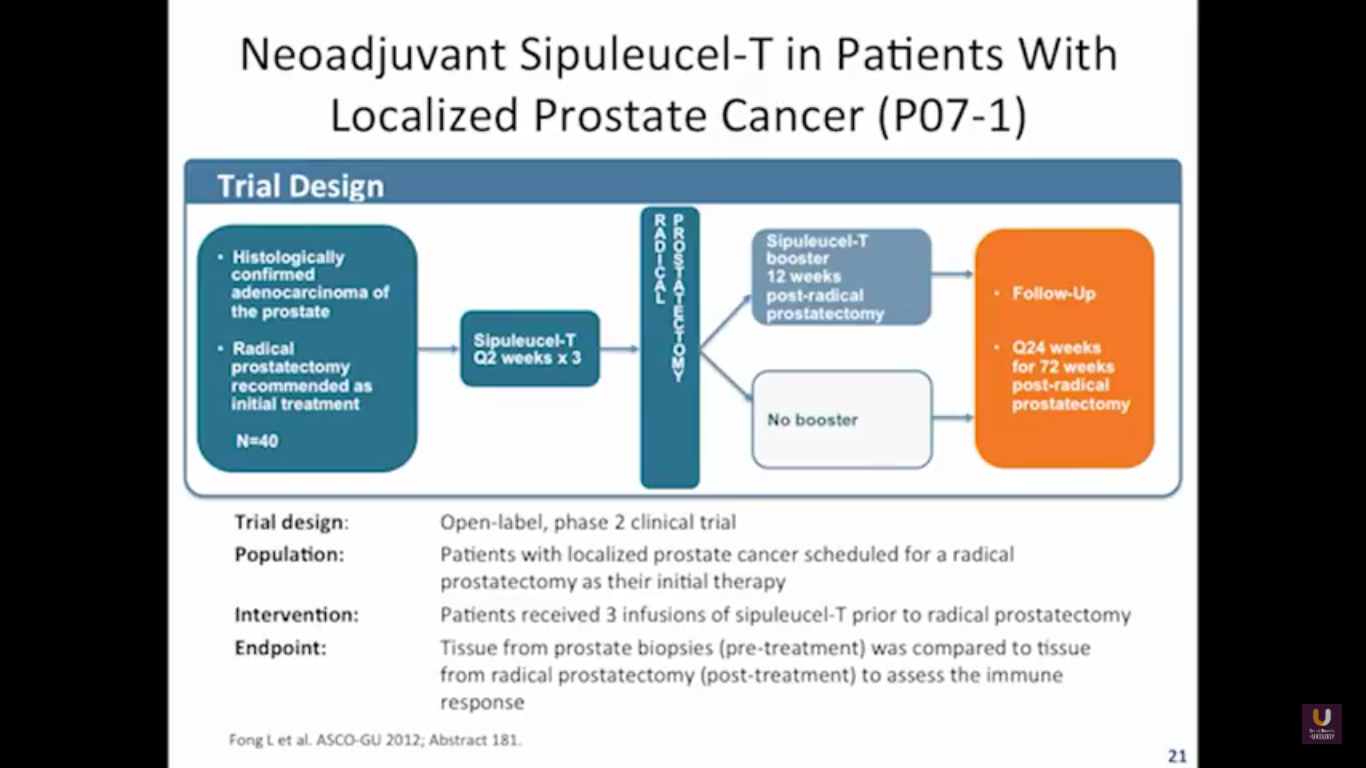
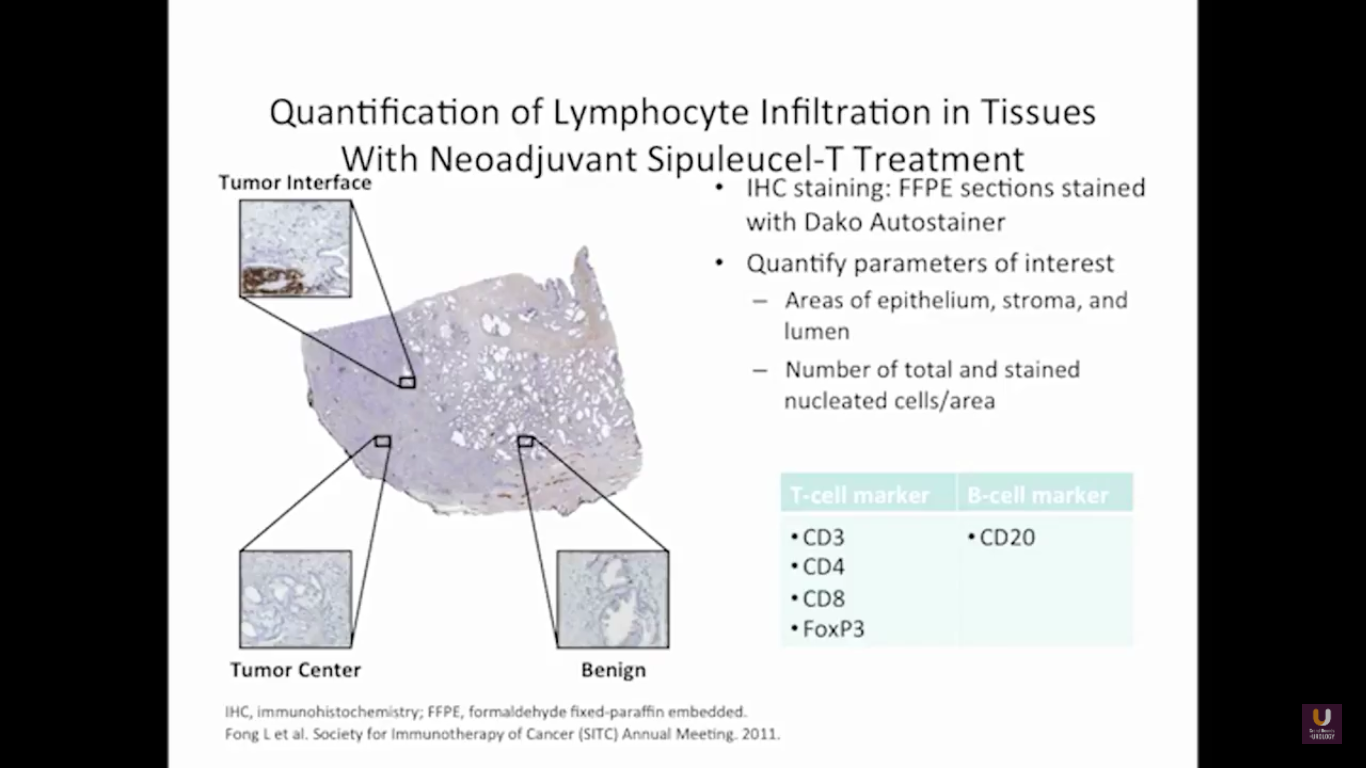
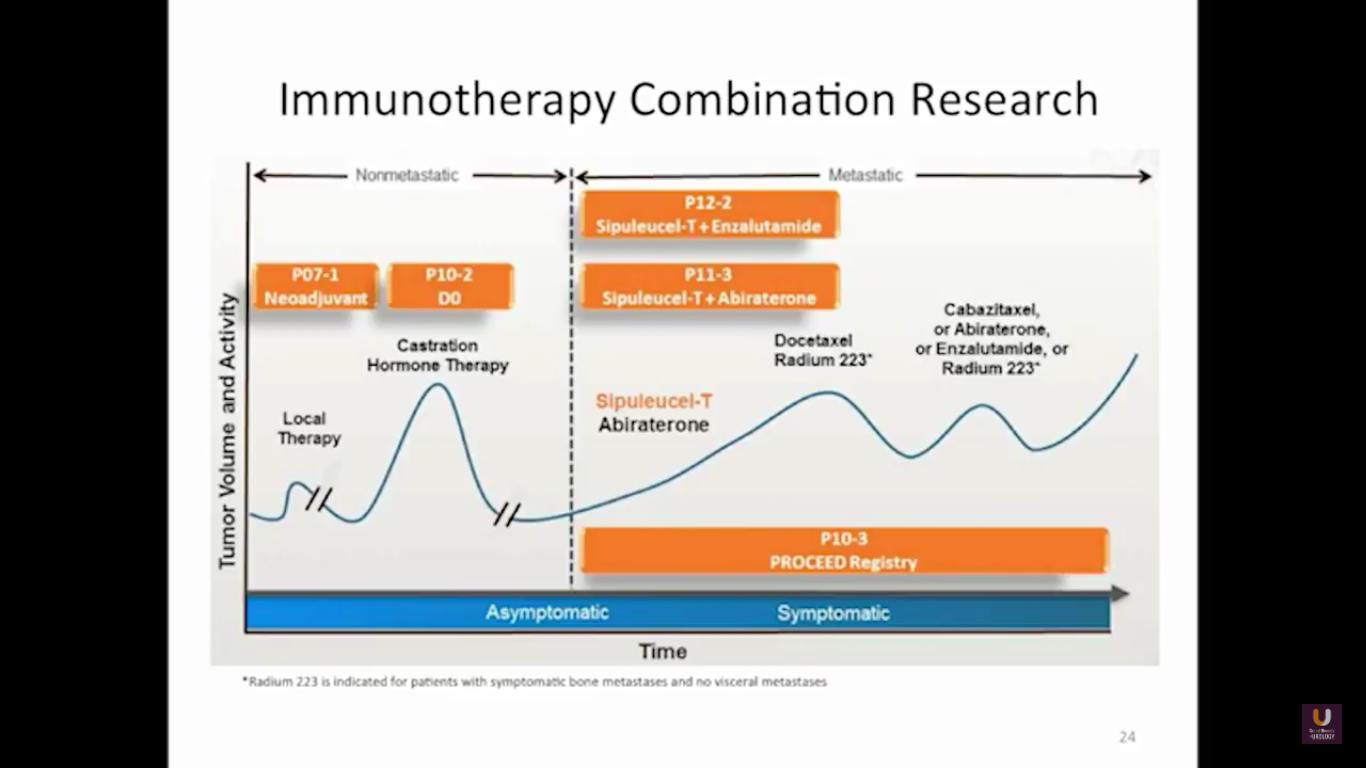
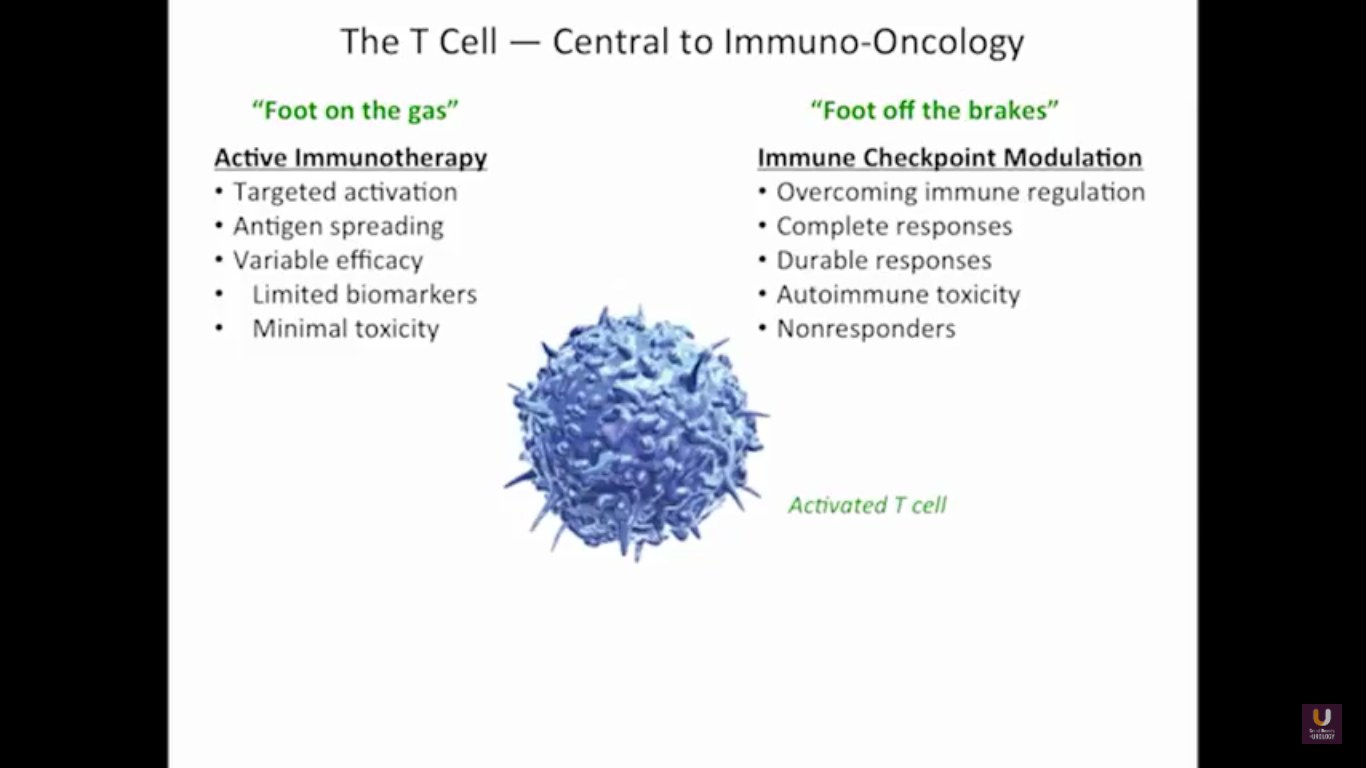
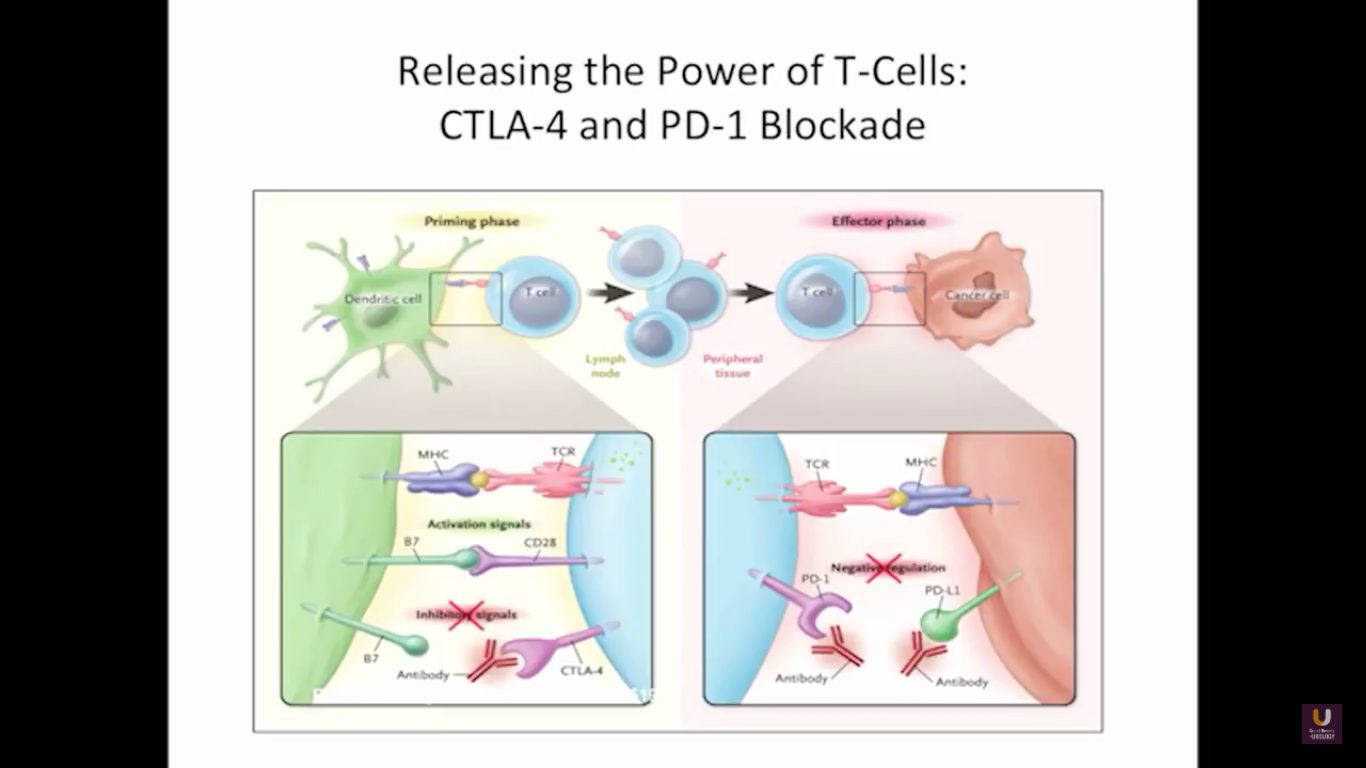
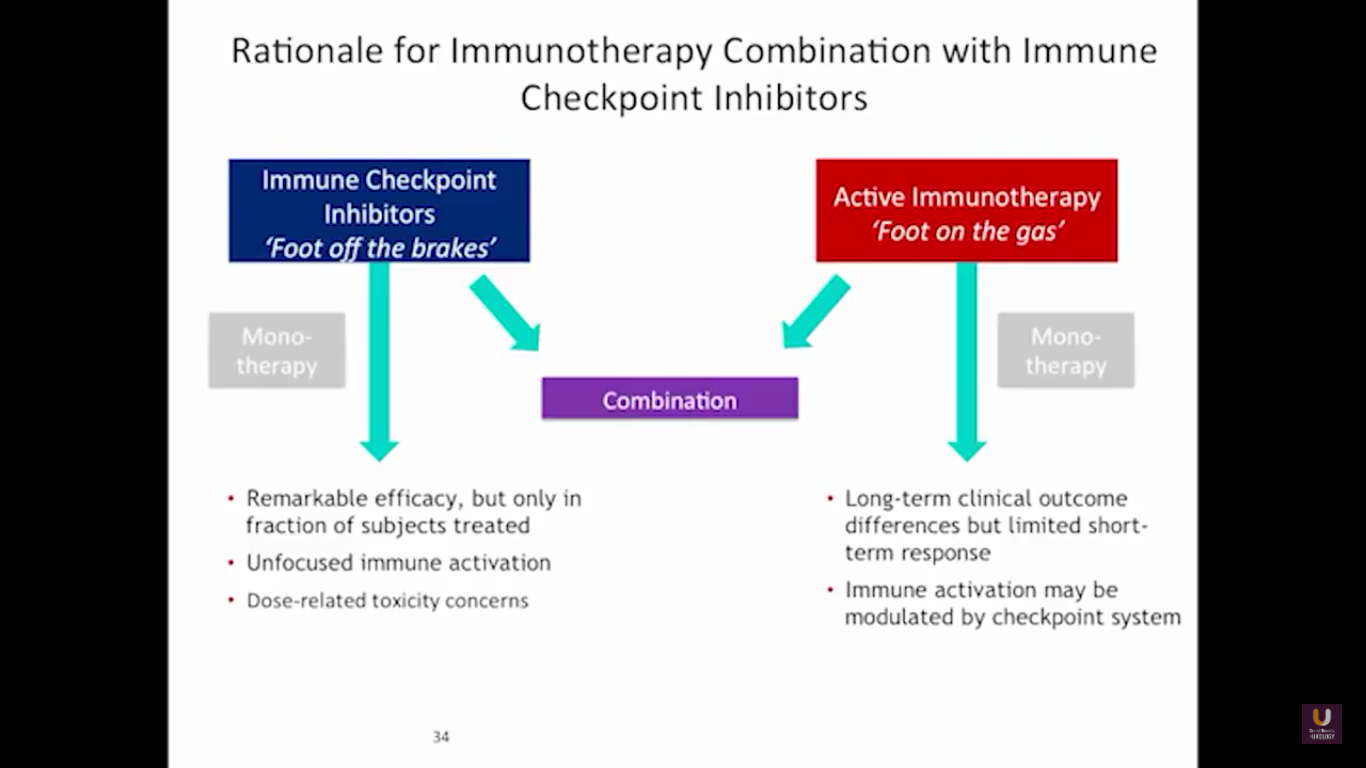
But what about this checkpoint modulation we keep hearing about? To answer that, first think about what the immune system does in general. The immune system has to recognize itself. It has to recognize you. So, with some autoimmune diseases, like rheumatoid arthritis, there’s disruption in that ability to recognize you as an individual. That’s where checkpoint proteins and checkpoint modulation, like CTLA-4 agents, like PD-1, PD-L1 blockers, come into the picture. These agents try to overcome immune regulation. If you think of active immunotherapy like a “foot on the gas,” checkpoint modulation is like a “foot off the brake,” because these checkpoint proteins slow down the system. They make it so our immune system cannot keep going unchecked.
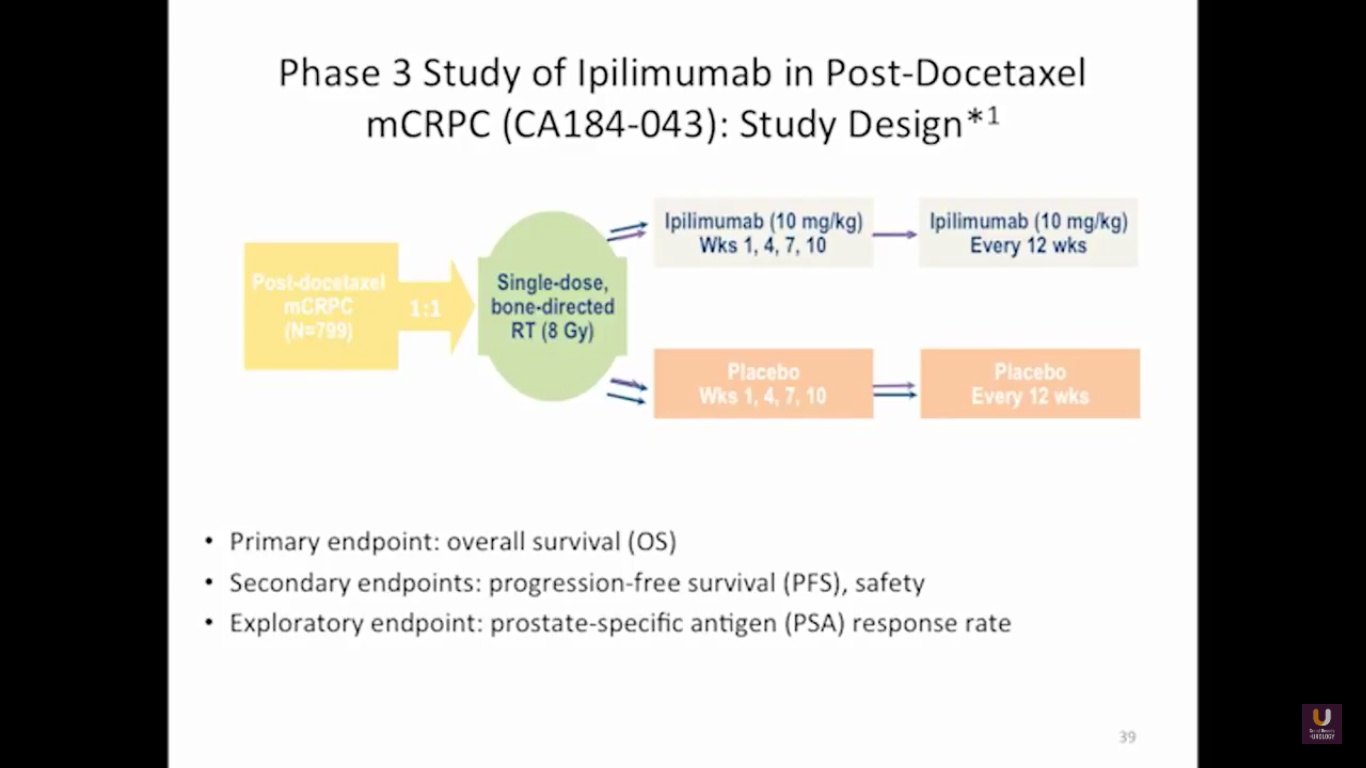
Looking at this abscopal effect, we can take into account this potential of using radiation. Also remember, we now have an approved FDA drug that’s an alpha emitter, and a radiopharmaceutical that has a survival benefit. This has the ability to deliver radiation intravenously. There are also a lot of trials receiving attention that potentially use this combination of immunotherapy with radiation.
Here are a couple studies I thought were interesting:


The rationale behind these trials is understanding the immune system, understanding the effects of radiation, and at the same time, trying to work with all these drivers, while still taking into consideration the antigen receptor.
ABOUT THE AUTHOR
Raoul S. Concepcion, MD, FACS, is the Chief Science Officer of U.S. Urology Partners in Nolensville, Tennessee. He was a resident in General Surgery and Urology at Vanderbilt University from 1984-1990, and later served as a Clinical Associate Professor of Urology there.
Dr. Concepcion’s clinical interests revolve around advanced prostate and bladder cancer management. He is a past President of LUGPA. Along with two other urologists, he founded CUSP, a urologic research consortium in the United States. Additionally, he is an advisor and/or speaker for many companies, including Dendreon, Pfizer, Astellas, Amgen, Cellay, and Janssen, and has served as editor for Urologists in Cancer Care.





