Dr. Laurence Klotz, MD, presented “Active Surveillance: From Biology to Bedside. Who fails, why, and how can we prevent this?” at the 2nd Annual International Prostate Cancer Update on January 27, 2018 in Beaver Creek, Colorado
How to cite: Klotz, Laurence. “Active Surveillance: From Biology to Bedside. Who fails, why, and how can we prevent this?” January 27, 2018. Accessed Dec 2024. https://Active-Surveillance-From-Biology-to-Bedside/
Summary:
Dr. Laurence Klotz, MD, argues that active surveillance should continue to be a standard of care for prostate cancer, as long as the urological community takes steps to improve screening methods and reduce overdiagnosis.
Reveal the Answer to Audience Response Question #1
The current AUA guidelines on the management of low risk prostate cancer state which of the following?
- A. Preferred treatment options include active surveillance, radical prostatectomy, or radiation
- B. MRI should be performed routinely in men considering active survillance
- C. Tissue based biomarkers have an important role in patient selection for active surveillance
- D. Active surveillance is the management strategy of choice for low risk prostate cancer
- E. PSA doubling time is a reliable trigger for intervention
Reveal the Answer to Audience Response Question #2
What is the main reason low risk patients fail?
- A. GG1/Gleason 3+3 metastasizes occasionally (although uncommonly)
- B. Misattribution of concurrent higher grade cancer (present but missed on biopsy)
- C. Gleason 3+3 dedifferentiates over time to higher grade cancer which metastasizes
- D. All of the above are important causes
Reveal the Answer to Audience Response Question #3
- A. Age
- B. PSA density
- C. Race
- D. MRI score
- E. Extent of cancer on biopsy
- F. All of the above
Active Surveillance: From Biology to Bedside. Who fails, why, and how can we prevent this? – Transcript
Click on slide to expand
Active Surveillance: From Biology to Bedside
I’m particularly happy to have the opportunity to give this talk because I know Dave Crawford, almost every single time I hear him speak, says we’ve got to get rid of active surveillance. It’s over; it’s dead. And I don’t think that’s entirely true. It’s going to stay with us although I agree with the basic concept that taking steps to improve screening and reducing over diagnosis and less low-grade cancer is laudable.
Active Surveillance for low risk PCa: What has changed?
This is not a new idea. Our first publication was 2002, and the basic concept began about 20 years ago. It was obvious to us that there were a lot of patients being treated with indolent disease, but there was no kind of described way to get around this at the time, and there was a strong sentiment that all newly diagnosed patients are being treated. So it’s now a new era. There’s no disagreement about the issue of over treatment, I would say. I saw the vote earlier.
Most people think even today with better understanding there’s still some over treatment going on. We know much more about the nature of occult, high-grade disease, which is the Achilles heal of the surveillance approach, but we’re getting a better handle on that. The predictive value of baseline parameters, PSA density, race, age, of course biomarkers, the flaws of PSA kinetics as a trigger, which was relied upon by most groups for the first roughly 15 years of this era, but not any more. Sensitive but not specific. The game changer of multi-parametric MRI, new modeling studies and longer follow up, and the last time I did a PubMed search on active surveillance in prostate cancer, 2,750 publications on this topic.
2018: What we know
So this is a consensus of what most people who are in this field agree upon. First, that we know much more about how the molecular genetics of Gleason 3 and 4 compare, and they are like night and day. And I’m going to go into that in a little bit of detail. The corollary of that is the metastatic potential of Gleason 3 is now acknowledged to be essentially zero. And I have asked many audiences over the years who has a case of a patient treated with prostatectomy so you don’t have the issue of misattribution of grade from the biopsy, where it’s pure Gleason 6 and the patient goes on to recur and metastasize. Almost no cases, and the ones that there are is a handful you have to wonder about. So I think this is a fair statement whereas as soon as you have Gleason 4, you have a real cancer, and we have mounting evidence for that, which I’ll review as well.
I think it’s well known now the Achilles’ heel of the consumer management of the Gleason 3 grade group 1 is that about 25% of those cases harbor higher grade cancer. Now, most of them are Gleason 3+4, and they also may not represent that much of a threat during the patient’s lifetime but tremendous efforts now to identify those cases earlier, and it’s become quite clear that the failures of surveillance were the patients who harbored the high-grade cancer at diagnosis in almost all cases.
In contrast, going from pure Gleason 6 to Gleason 7 or 8 over time, biologic progression is actually quite rare. We know there are cases, probably around 10% that are Gleason 6 who harbor molecular genetic features that are adverse, and this is the basis for the Prolaris, and OncoType Dx, and clearly those have a role. There’s a lot of really important data. Recently we started to hear about this earlier on germ line mutations, particularly DNA repair mutations, and that has very significant ramifications for those patients who harbor the mutations who are contemplating surveillance, and then, of course, the complementary roles of MRI and biomarkers.
Finding the wolf in sheep’s clothing: 2 different species of wolf:
I mentioned the two species of wolves in sheep’s clothing, the misattribution of higher grade cancer. You see the Gleason 6, but the patient has got something worse, and this is the real problem, around a quarter of cases. There’s very little known about this rate of biologic grade progression, which is surprising considering how fundamental an issue it is. How likely is the guy with, say, extensive Gleason 6 to progress to higher-grade cancer? There’s three papers on this. One is from our group. One is from Hopkins, and the best one is this modeling study from Ruth Etzioni’s group. They all calculate roughly 1 to 2% per year of grade progression usually in a field of large volume Gleason 6. So this means in the short term it’s not really an issue. In the long term you’re following patients 10 or 15 years. They have a say 15% chance of grade progression, again not necessarily the lethal cancer, but they need long-term follow-up.
There are virtually no well documented cases of pathologically proven Gleason 6 cancers that have metastasized
This issue of non-metastasizing Gleason 6 very fundamental. Two studies, well known, just briefly the Eggener study of 12,000 Gleason 6 treated with surgery. They basically did not find one patient with true Gleason 6 confirmed on re-review who metastasized. They report 2 in 1,000, but they were all upgraded eventually, and the same thing with the Ross paper, 14,000 cases, essentially no pure Gleason 6 that metastasized to the lymph nodes in all those cases. So this is pretty compelling evidence I would say. And even if it is 0.1%, it doesn’t mean that you should alter your management based on that.
Gleason 3 lacks hallmarks of cancer
And I mentioned, the very comprehensive understanding now of how 3 compares to 4 in terms of the pathways of oncogenesis, and if you look at proliferation, apoptosis, angiogenesis, response to growth signal inhibition, and so on, it is remarkable how Gleason’s low-power view of the – -disaggregates cells into those with very normal looking molecular genetics, and those with abnormal.
Now, as you would expect there are some exceptions. One exception is PTEN loss, which is a key molecular event in the pathogenesis of invasion of metastasis. 10% of Gleason 6 reported to have PTEN loss versus around 90% in higher grade cancer.
Genomic alterations quantitatively, not qualitatively different between grades
This is a study from Mark Rubin’s group, that’s Grade group 1 on the left, grade group 5 on the right, and it shows the mutational burden that increases dramatically as you go from low grade to high grade, so you see high-grade prostate cancer has an absolute plethora of mutational events, and on the right you can see here this one is PTEN loss. So it does occur, but as an isolated event it doesn’t seem to have much in the way of clinical significance. Again, these patients don’t metastasize.
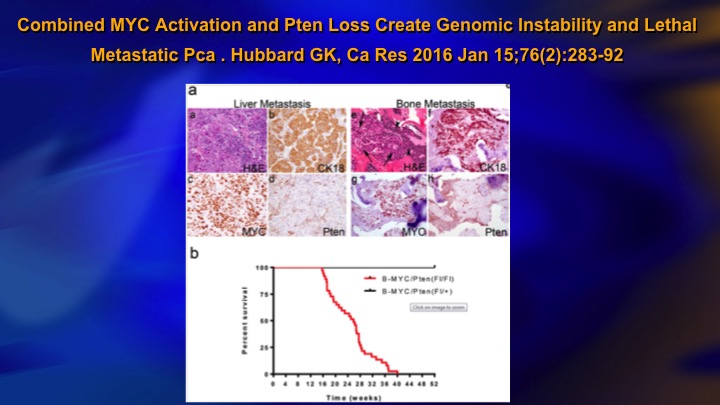
Combined MYC Activation and PTEN Loss Create Genomic Instability and Lethal Metastatic Pca
One reason for this, this is a study published last year, a pre-clinical study, but it showed basically for PTEN loss to be—to result in metastasis, you needed coexistent MYC activation, and you can see the top line is MYC activation or PTEN loss and this line all the animals died is the combination. So it makes sense. You have the odd, sporadic, mutational event that does not translate into metastasis because you need a combination of events, a kind of two-hit hypothesis, to really get lethal disease and you basically do not see that in low-grade cancer.
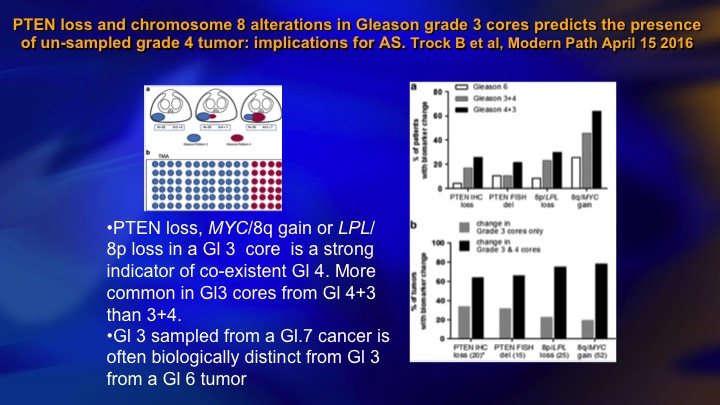
PTEN loss and chromosome 8 alterations in Gleason grade 3 cores predicts the presence of un-sampled grade 4 tumor: implications for AS
It turns out that those patients who have Gleason 6 who do have PTEN loss, tend to be the cases where there’s coexistent higher-grade cancer, so this just shows—this is a large group of patients from Hopkins where they had Gleason 6 or 7, and they teased out the Gleason 6. And this is the incidence of PTEN loss in the 6, the 3+4, the 4+3. And it goes across here. The PTEN loss tends to occur in Gleason pattern 3 when there is pattern 4 present. So there’s two possible explanations for that finding. One is that you are seeing the three on its way to a 4. And so you have four present, and it just shows the maturation. The other sort of intriguing idea, which is getting a lot of air time recently is this intracellular communication through the means of extracellular vesicles, extracellular vesicle transfer.
Implications of Extracellular Vesicle (EV) Transfer on Cellular Heterogeneity in Cancer
It turns out that particularly these high grade prostate cancer cells are shedding. Each cell can shed thousands of these extracellular vesicles per day, which harbor biologic material including micro-RNA, and messenger RNA, and protein, and they may result in silencing of genes in the cells that take up these extracellular vesicles. It’s been shown in the animal model the high grade cancer will induce a more aggressive phenotype in a low-grade cancer, so high-grade, low-grade, and it’s kind of like the good cancer has gone into a bad neighborhood and been adversely influenced. So this has a lot of implications for a number of clinical scenarios and certainly is one explanation that is kind of counter intuitive.
Spontaneous regression of cancer likely more common than we think
Another thing, we don’t think about too much is actually spontaneous regression. A third of low-grade prostate cancers rebiopsied are negative, and we always assume that is just sampling, it’s small volume, you miss it on the next biopsy. In breast cancer, it’s widely acknowledged that spontaneous regression due to lack of telomerase, inborn senescence, lack of VEGF, score of reasons, and the cancers disappear, and it’s quite plausible that this is a—it’s certainly widely acknowledged in breast cancer and probably in our area.
Most guidelines differentiate between very low risk and low risk based on cancer volume
So if Gleason 6 is not metastasizing, why do we worry about larger volume? The reason, and every guideline differentiates between very low risk, which is Epstein criteria in most cases, and just low risk, which is higher volume Gleason 6, and it does make sense because the volume is a marker for the presence of co-existent higher-grade cancer.
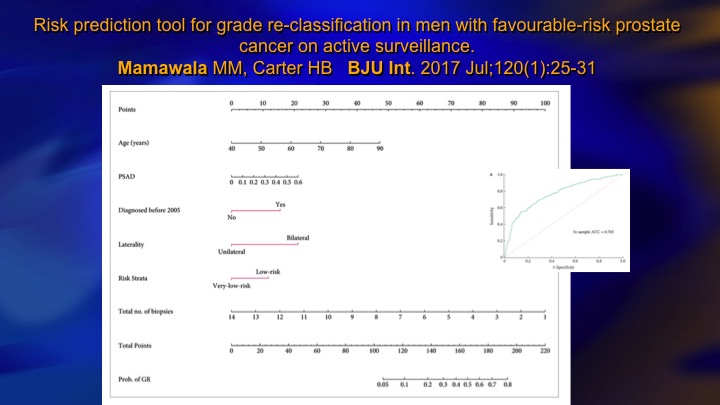
Risk prediction tool for grade re-classification in men with favorable-risk prostate cancer on active surveillance
This is one recent study that incorporates PSA density and laterality, if it’s bilateral it’s higher volume, and a number of positive biopsies, and very low versus low risk, so there’s clinical parameters that allow you to very well predict based mainly tumor volume or surrogates for tumor volume like PSA density who is going to—who harbors the higher-grade cancer.
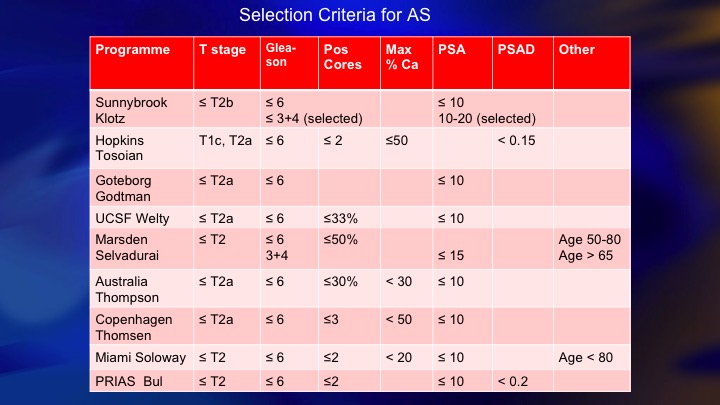
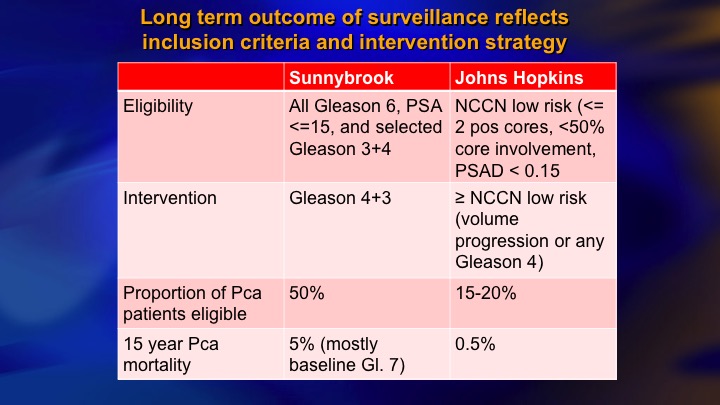
Selection Criteria for AS
This is a summary of 9 prospective series of surveillance in the literature. There is now more than 10,000 patients including the huge PRIAS multinational collaboration, but I just want to compare these two: so ours and Hopkins, and the reason these are two of the largest and most mature. They started around the same time. They both have well over 1,000 patients. But we took completely different perspectives, and our perspective was an inclusive one. We offered surveillance to everyone we thought was a reasonable candidate, including Gleason 7, particularly small volume 4, and we weren’t too worried about the PSA being less than 10. Bal Carter at Hopkins for a variety of reasons, philosophy, maybe fear of litigation, who knows, took a very conservative, restrictive approach—I won’t say very conservative. Let’s say conservative, restrictive approach, just Epstein criteria and they treated for volume progression of Gleason 6.
So the whole concept of is it just low testosterone that’s leading to these abnormalities, or is low testosterone as a result of ADT being modulated through some of the actions of the multitude of actions of FSH.
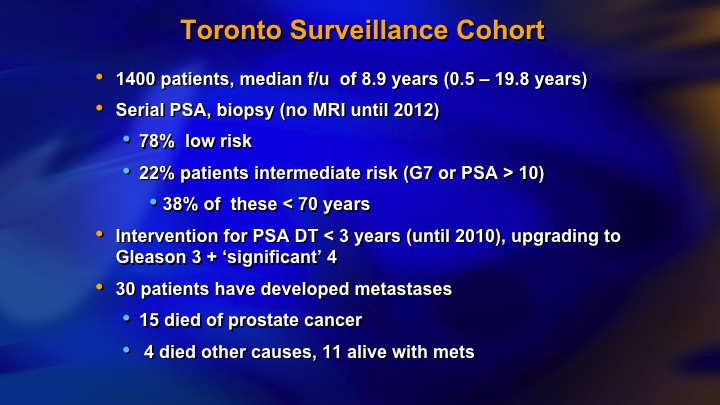
Toronto Surveillance Cohort
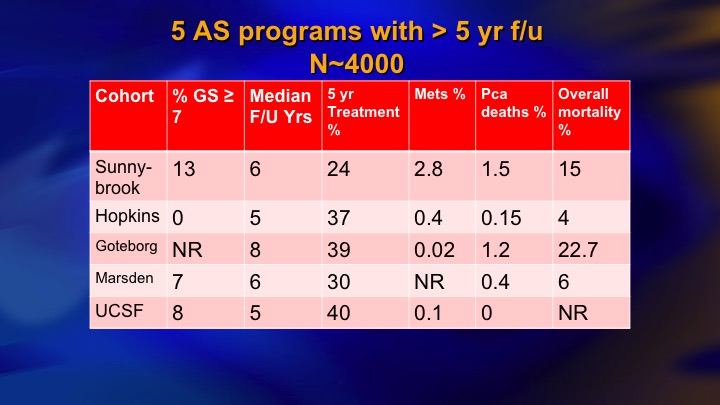
And this is our kind of last data. We had 22% intermediate risk, now 1400 patients, 30 metastasizing patients, 15 died of prostate cancer.
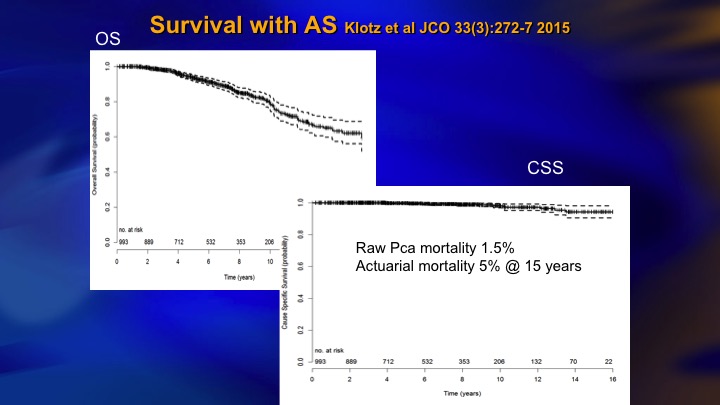
Survival with AS
This came out about two years ago. 5% actuarial mortality although the raw rate is 1.5%, and for about 6 months I was very proud of this paper. 5% mortality with 15 years follow up in a prostate cancer cohort is pretty good.
Hopkins AS long term outcome: overall mortality and Pca
Then my ebullience was destroyed by this paper from Hopkins, where also 15 year outcome, 0.5% mortality. So there clearly was a benefit to this more restrictive approach that they took.
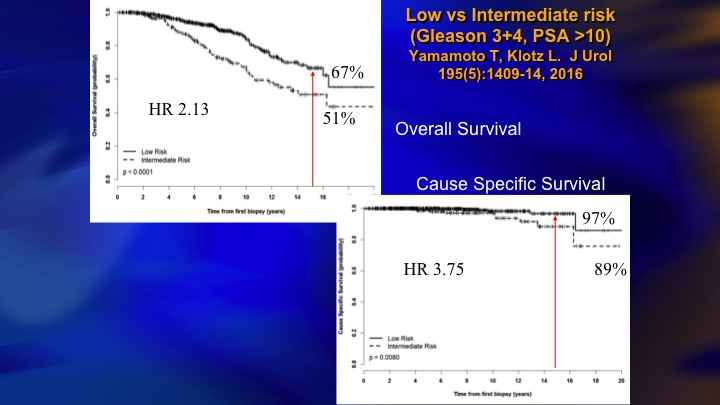
Low vs Intermediate Risk
So we looked at our data to try and figure out why did we have a higher mortality, even though 5% is, I would say not bad, but obviously room for improvement. And it basically was all of the intermediate risk patients at baseline who accounted for the deaths, the hazard ratio, four times greater for the intermediate risk.
Recursive partitioning analysis: Metastasis free survival by risk group
And it wasn’t the PSA. It was all the presence of Gleason 4 at baseline. This is the recursive partitioning analysis. Gleason 6 over here. They all have like 90 to 95% actuarial 15-year metastasis free rates. But in the Gleason 7 here, even in the best group, small volume, PSA less than 10, 3+4 30% calculated metastasis rate. So that was a wake-up call.
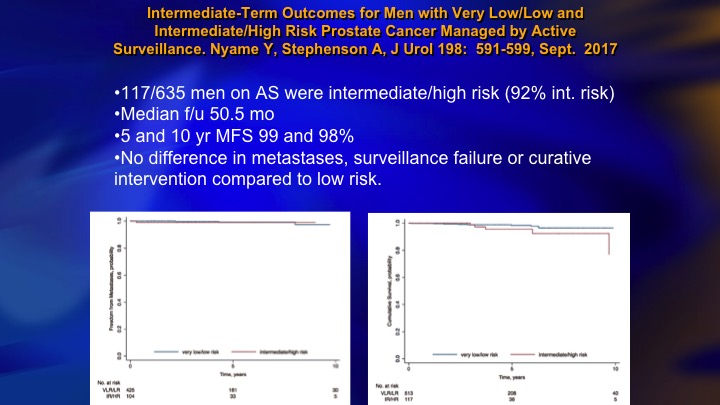
Intermediate-Term Outcomes for Men with Very Low/Low and Intermediate/High Risk Prostate Cancer Managed by Active Surveillance
There’s a lot of data that has come out recently on this issue of the safety of surveillance for Gleason 7. This is actually the wrong slide deck. I don’t know this is—I had some other data in here, which I don’t think is in this deck. Let me just see, it’s not. There is a paper from Hopkins that looked at Gleason 3+4 who had radical prostatectomy, and these were favorable 3+4’s. I’m sorry the slide’s not here, just published in JAMA Oncology a month or so ago. 25% of the favorable 3+4’s had adverse pathology meaning predominant 4+3, and most strikingly there was no predictive factors to identify who the high-risk groups were. So this was a very sobering study demonstrating that any 3+4 at diagnose was associated with a relatively high risk of harboring high-grade cancer.
Now, in contrast you have this group from Cleveland Clinic, Andrew Stephenson, 117 intermediate risk on surveillance, 5 and 10-year metastasis free survival, 99%, no difference in metastases, surveillance failure, or curative intervention compared to low-risk. So this is a controversy at the moment. There is clearly intermediate risk patients who are candidates for surveillance, and lots of these patients do very well, but it is riskier. It is a lot riskier than the Gleason 6, particularly the ones who are well-characterized, and caution is warranted.
Long term outcome of surveillance reflects inclusion criteria and intervention strategy
To make a long story short, comparing the Sunnybrook and Hopkins kind of initial approach as inclusive, restrictive, 50% eligible versus about 15 to 20% of newly diagnosed eligible, so that is the down side of restrictive approach. The benefit, a fewer deaths. There’s clearly been a convergence. I think the Hopkins’ group acknowledges that most Gleason 6 are candidates, and we’re much more cautious about the intermediate risk than we were.
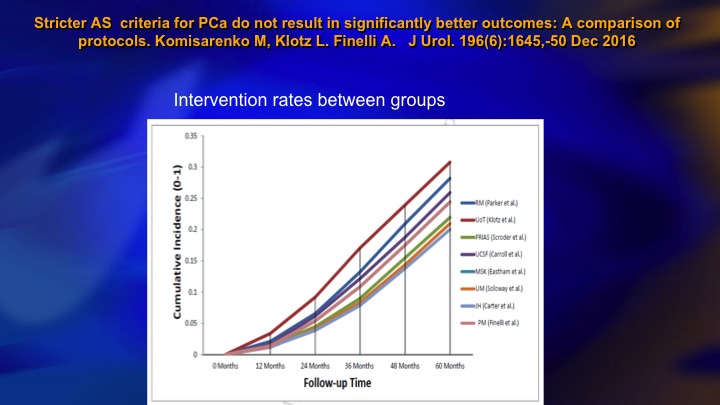
Stricter AS criteria for Prostate Cancer do not result in significantly better outcomes: A comparison of protocols
This is another publication from our group that compared nine different surveillance cohorts as to the intervention rate. Hopkins is down here. We’re up here. We’re a little higher, but what is striking is this all varies between about 20% and 30% intervention rate at five years. So the interesting thing is it seems no matter what your basic eligibility are, whether you are stringent or inclusive the intervention rate remains about the same.
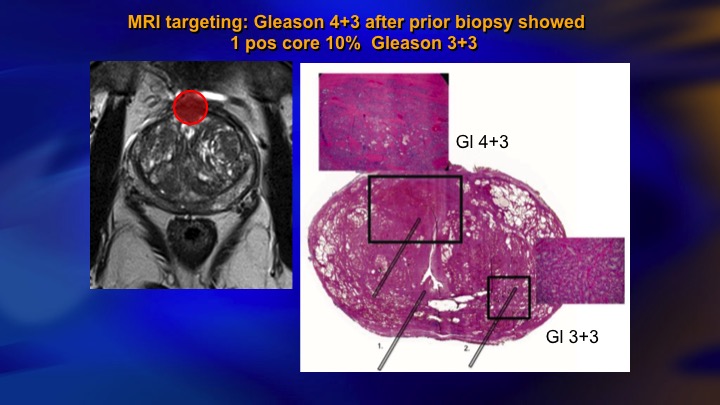
MRI targeting: Gleason 4+3 after biopsy showed 1 positive core 10% Gleason 3+3
MRI we’re going to hear some more about, but it’s obvious that this is having an impact. This is a typical case of a guy with mild elevation of PSA, systematic biopsies, we don’t do template, but showed a single core 10% Gleason 3. In the old days, we would have just rebiopsied him a year later. Today he has an MRI. There is the lesion there. You would never hit that with a systematic biopsy. There it was a Gleason 4+3. There’s the radical prostatectomy specimen. As is often the case, the actual lesion was underestimated on the MRI although it was identified. Here is the microfocus of Gleason 6. So this is an anecdote, but I mean clearly this is going to make how we do things better.
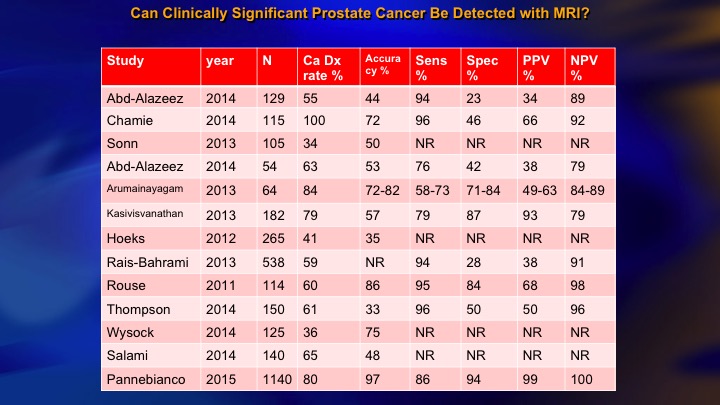
Can Clinically Significant Prostate Cancer Be Detected with MRI?
This is a summary of the 15 or so studies that are out there that look at the accuracy with which MRI can find higher-grade cancer, and you see the NPV over here of all of these studies. If the MRI is negative, how likely are they to have disease? And there is a big range. I mean we want this to be 90 to 95%. But the range is from about 80 or less to 100. The Panebianco study, not one MRI negative with clinically significant cancer in about 1,000 patients. Frankly, I think it is literally incredible. It is not plausible, and we have seen many more than that.
NPV of MRI: Meta-analysis from EAU Guidelines Panel
One of the important considerations about this is the NPV is a function of underlying risk. This is a recent publication from the EAU guidelines panel that just emphasized if you have a high-risk population the NPV of MRI is relatively low, around 65 to 70%. In a low-risk population, it’s more like 90%. So the NPV is a function of risk, and you have to keep that in mind when you are looking at your patient’s negative MRI and deciding whether to go further.
ASIST Trial Summary
We did one study that’s relevant. It’s just under consideration for publication by European Urology, 275 men on surveillance randomized between MRI with targeted biopsy and systematic biopsy and systematic biopsy alone for their confirmatory biopsy.
Artemis—MR/ultrasound fusion biopsy system
We used the Artemis device, which was one of the issues. We bought three of these devices with the funding for the trial, and next slide.
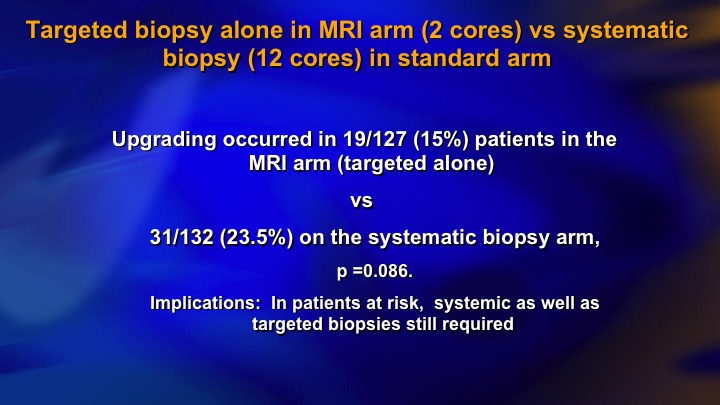
Upgrading
I just want to basically show some brief results. We were hoping to show that if you did targeted biopsies, you would find as much intermediate risk cancer, as if you did systematic biopsies, and we didn’t. And if you look here, we had three different sites. So the middle site was the largest one, and if the patients had a PIRAD 3, 4, or 5, one in three had significant cancer, at the largest site. At the other two sites, which didn’t accrue as many patients and clearly didn’t have as much experience with the Artemis device, the positive predictive value of a positive MRI finding was 10% or less. 90% of those patients having targeted biopsies had no evidence of significant disease. And furthermore, the targeted biopsy did not find as much.
Targeted biopsy alone in MRI arm (2 cores) vs systematic biopsy (12 cores) in standard arm
So this partly reflects learning curve. It partly reflects I think limitations of the targeted biopsy approach. Not all significant cancer shows up on MRI and at this point I mean in a low-risk group I think it’s quite effective. In the higher-risk group, they still need systematic biopsies.
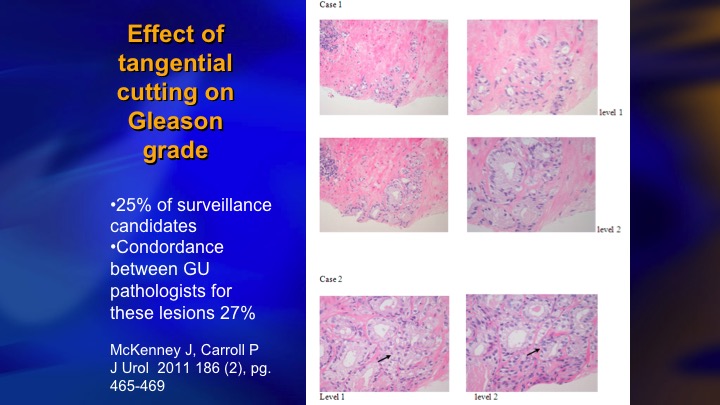
Effect of tangential cutting on Gleason grade
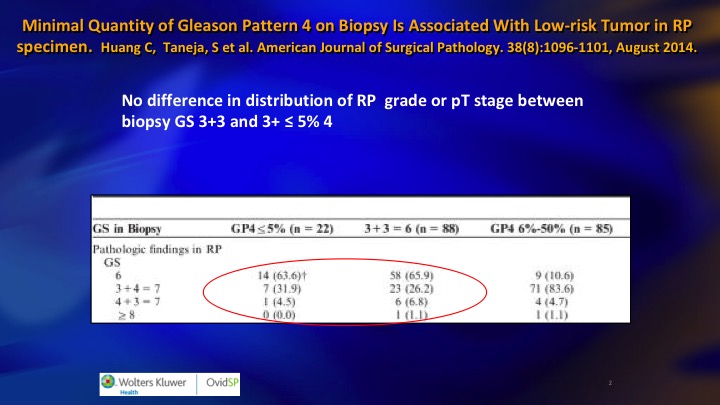
One of the important considerations in identifying intermediate risk patients who are candidates for surveillance is the key group who have 5% or less Gleason pattern 4, and I don’t know if this is well recognized. Patients with very small amounts of pattern 4 are very prone to have artifactual upgrading because of tangential cutting of a Gleason 3 – – . The needle misses the lumen and all you see is a clump of cells, and a pathologist who is trying to quickly get through a large number of cases will call it a Gleason 3+4. They tend to be just a small amount of 4, and one of the proofs that this is a real phenomenon, next slide is this is one paper from NYU, Sumeer Taneja that showed the cases who had less than 5% pattern four Gleason 3+4, less than 5% on biopsy had exactly the same distribution of radical prostatectomy pathology as 3+3.
Minimal Quantity of Gleason Pattern 4 on Biopsy is Associated with Low-risk Tumor in RP specimen
In other words more than 60%, 65% were only 3+3. The Gleason 4 disappeared, and so these are a special group. More than 5% or certainly more than 10% I think active surveillance at the moment probably not the best strategy.
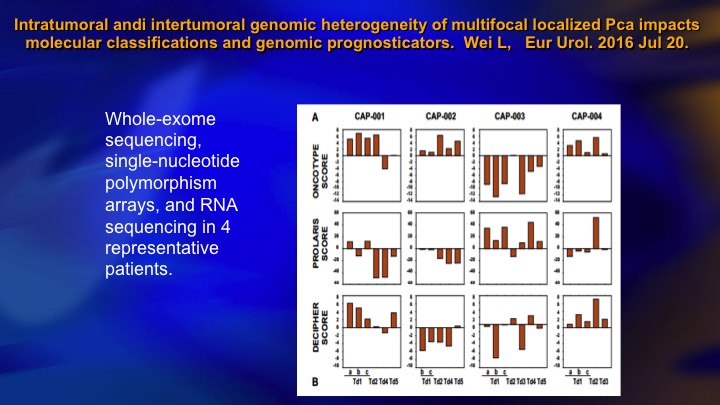
Intratumoral and intertumoral genomic heterogeneity of multifocal localized prostate cancer impacts molecular classifications and genomic prognosticators
The biomarker obviously also play a major role. The biomarkers suffer from one limitation, which we’re intimately familiar with in prostate cancer, which is heterogeneity. So this was a gorgeous study where these authors took a small group of patients, it’s four different patients, these patients all had multi-focal cancer, so each bar here represents one small cancer in the same prostate. This patient, for example, had six different cancers. They did whole exome sequencing on these micro-dissected cancers, and then ran the panel of Oncotype, Prolaris, and Decipher, for each of these cancers, and what do you see? You see a lot of heterogeneity. You see high scores, low scores, positive, negative, they tend to trend in the same direction, but remember these are quantitative assays, and there’s heterogeneity, so what does it mean on this patient if this is the read out of Oncotype and this is the rest of his cancer? I think that is a challenge for the biomarker area. They obviously have a lot of predictive value, but it’s something to consider.
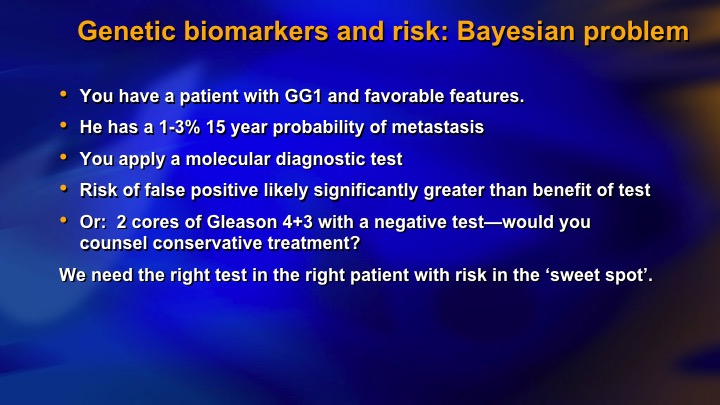
Genetic biomarkers and risk: Bayesian problem
One more aspect of this is what you could call the Bayesian problem. You have a patient with Grade group 1, favorable features. We know he’s got a 3% chance of metastasis at 15 years, even less if he is at Hopkins. You apply the molecular diagnostic test. Let’s say it’s 90% accurate. You have a 10% chance of a false positive, which is between 3 and 10 times higher than the patient’s actual risk of having significant cancer or metastasis. So there’s a real risk of a false positive in the very low-risk patient, and by the same token in the very high-risk patient, you have a 4+3. Dave mentioned doing the molecular tissue-based molecular assay in these patients but I would have a lot of difficulty counseling conservative management in a 4+3 no matter what the tissue-based molecular assay was. So you need the right test and the right patient with the right risk level, and I’m just going to go to the last slide if I can get this thing to work. The final point I want to make is about this incredible data on DNA repair germline defects.
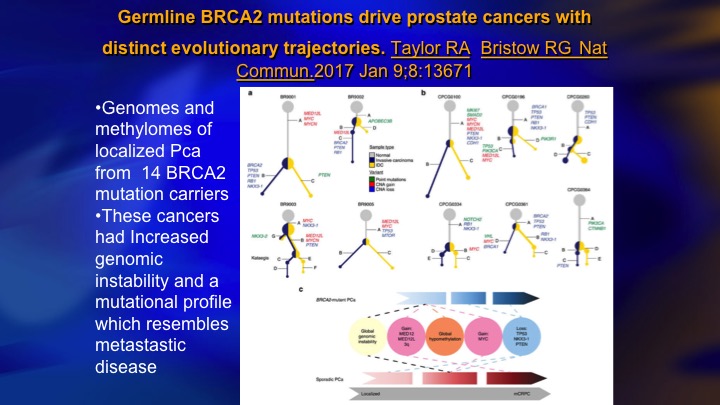
Germline BRCA2 mutations drive prostate cancers with distinct evolutionary trajectories
What we have learned is that the mutational burden in patients who have BRCA2, BRCA1, ATM type DNA repair defects accumulates with incredible rapidity. What happens is as soon as these patients have the initial event, it’s like there’s an explosion of mutational events, and this study took a small group of patients who are BRCA2 carriers, and they found in the patients with localized prostate cancer their genetic burden was the same as metastatic CRPC, and you can sort of see this schematically along the bottom, the sporadic cases where they start to get all of these different hypomethylation amplification, etc. late. In BRCA2, they get them very early. I don’t even know if there is such a thing as Gleason 6 with a BRCA2 mutation because the ed is that these patients progress so rapidly, but I think when you do find a surveillance candidate who’s got a germline mutation, and you need to suspect it from family history and so on, today I think that patient should be treated.
IPCU
Question from the Audience:
Very nice talk. Thank you very much. You kind of showed the down sides to the tumor markers, MRI, et cetera. What is your practice right now when you find a low-risk patient? Do you use these standards or not?
Dr. Klotz’s Answer:
Yeah. So, we’ve adopted MRI. It’s partly availability. In Canada, there’s a few more barriers to using the tissue-based biomarkers, not just cost, but the labs are in the States, the tissue has to be sent out of country, et cetera, so they haven’t really caught on in our country. MRI has caught on so our basic strategy is every newly diagnosed surveillance patient gets an MRI and a targeted biopsy. The issue is whether they still need the confirmatory systematic biopsy if the MRI is negative, and based on our recent data, we think, yes, the MRI is not ready to completely replace the—if it’s negative to replace the systematic biopsy although in the very low-risk patient a single microfocus, low PSA density and so on, there’s probably the yield of the systematic biopsy is going to be low.
The biomarkers we do kind of infrequently, and mostly in the patients where there’s a discrepancy, for example Gleason 7 with a negative MRI. But not as a routine.
ABOUT THE AUTHOR
Laurence Klotz, MD, is the former Chief of Urology at Sunnybrook Health Sciences Centre and former President of the Urological Research Society and the Canadian Urological Association. He currently serves as Professor of Surgery at the University of Toronto and holds the Sunnybrook Chair of Prostate Cancer Research. Dr. Klotz was the Founding Editor-in-Chief of both the Canadian Journal of Urology and the Canadian Urology Association Journal, and is now Editor Emeritus of the CUAJ. He is the Founder and Chairman of the Canadian Urology Research Consortium (CURC), and is also the Chair of the Global GU Oncology Group.
Dr. Klotz obtained his medical degree from the University of Toronto and completed his residency at the University of Toronto Gallie Program in Surgery. He was a fellow at Memorial Sloan Kettering Cancer Center in New York in uro-oncology.
Dr. Klotz is a widely published uro-oncologist with over 350 publications and several books. His main research interest has been prostate cancer. He has served on the boards of many medical/scientific organizations and journals, including the SUO, Prostate Cancer Canada, the journals Prostate Cancer and Prostatic Diseases, Brazilian Journal of Urology, Italian Journal of Urology, and World Journal of Urology.
Dr. Klotz was awarded the Queen’s Jubilee Medal for meritorious public service in 2012, and the University of Toronto Department of Surgery Lister Prize and the Society of Urologic Oncology Medal in 2013. He received the Harold Warwick Award from the Canadian Cancer Society for ‘outstanding contributions to cancer control’ in 2014. He received the Order of Canada in 2015, and the Richard Williams Award from the AUA in 2016. He received the Dean’s Lifetime Achievement Award from the University of Toronto in 2017.