Neal D. Shore, MD, presented “Navigating the M0 Space- Results of RADAR III” during the 29th Annual International Prostate Cancer Update on January 26, 2019 in Beaver Creek, Colorado.
How to cite: Shore, Neal D. “Navigating the M0 Space- Results of RADAR III” January 26, 2019. Accessed Dec 2024. https://dev.grandroundsinurology.com/navigating-the-m0-space-results-of-radar-iii/
Navigating the M0 Space- Results of RADAR III- Summary:
Neal D. Shore, MD, reviews the recently-published guidelines focused on next generation imaging for early detection of disease progression in M0 prostate cancer in the Prostate Cancer Radiographic Assessments for Detection of Advanced Recurrence III (RADAR III). He also reviews the goals and conclusions from RADAR I and II.
RADAR I
In 2014, a multidisciplinary group including E. David Crawford, MD; Nelson N. Stone, MD; Phillip J. Koo, MD; and other Grand Rounds in Urology faculty, published RADAR 1. This project was a review of the available imaging guidelines for metastatic prostate cancer at the time. Its goal was to identify optimal strategies for early identification of metastases in newly diagnosed prostate cancer, biochemical recurrence patients, as well as M0, or patients with non-metastatic castration resistant, prostate cancer.
The era’s standard imaging modalities consisted of Technetium 99m scintigraphy and full-body computerized tomography (CT). Guidelines still categorized plain radiography, MRI, and sodium fluoride positron emission tomography (PET) as additional tests.
Conclusions stated that inconsistency in literature and guidelines for advanced prostate cancer imaging contributed to the underdiagnosis of metastatic disease. The RADAR I group developed guidelines to promote a solution to this unmet need.
RADAR II
Shortly thereafter, a group conducted RADAR II with the purpose of providing a consensus on sequencing, combinations, and therapeutic layering for CRPC. Conclusions from this iteration stated that metastatic CRPC (mCRPC) management remains challenging despite advancements in therapy. The group considered it best to manage mCRPC with multiple unique and complementary agents. However, this creates concerns of cross-mutations and aberrations, which leads to more tumor burden, and ultimately, patient death.
The RADAR II Working Group advocated for optimizing treatments using the patient’s molecular character/genotype, as well as clinical characteristics. They also maintained that clinical trials were integral for providing evidence regarding efficacy, safety, tolerability, and patient identification for combination regimens.
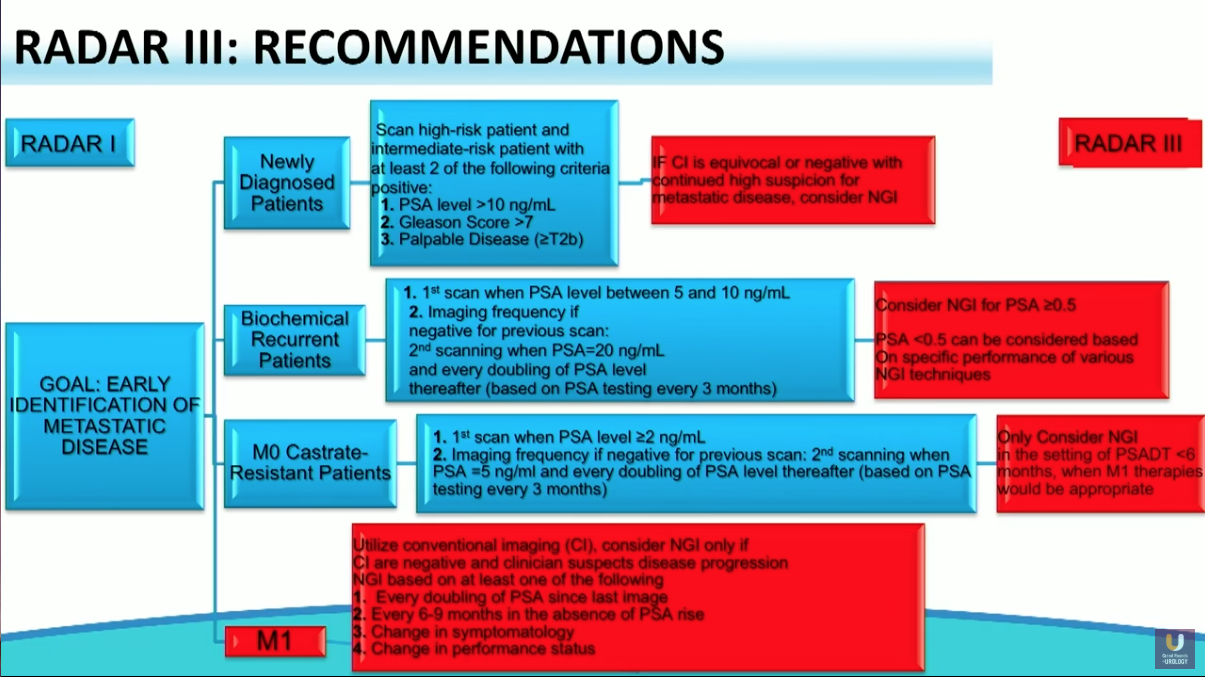
RADAR III
RADAR III, published in August of 2018, focuses on understanding the potential use of novel imaging modalities and integrating them into clinical practice and decision-making algorithms. This iteration also suggests redefining the terminology of CRPC to endocrine-resistant prostate cancer (ERPC).
In addition, RADAR III updated the recommendations set forth in RADAR I and II. The updated recommendations state that conventional imaging continues to have a role in diagnosing prostate cancer. However, they express favorability toward next generation PET technologies, especially in patients with biochemical recurrence, and promote improved global accessibility of prostate specific membrane antigen (PSMA) agents.
The studies evaluated during RADAR III showed that next generation PET imaging outperforms conventional imaging in terms of sensitivity in visualizing advanced prostate cancer. Still, there is a lack of prospective, phase III trial data and ongoing safety data for these novel imaging modalities. Further studies will warrant updates to these recommendations.
About the International Prostate Cancer Update
The International Prostate Cancer Update (IPCU) is an annual, multi-day CME conference focused on prostate cancer treatment updates. The conference’s faculty consists of international experts and caters to urologists, medical oncologists, radiation oncologists, and other healthcare professionals. Topics encompass prostate cancer management, from diagnosis to treating advanced and metastatic disease. Dr. Shore presented this lecture during the 29th IPCU in 2019. Please visit this page in order to learn more about future IPCU meetings.
ABOUT THE AUTHOR
Neal D. Shore, MD, FACS, is the Medical Director for the Carolina Urologic Research Center. He practices with Atlantic Urology Clinics in Myrtle Beach, South Carolina.
Dr. Shore has conducted more than 350 clinical trials, focusing mainly on genitourinary oncology, and serves on the executive boards of the Society of Urologic Oncology and the Bladder Cancer Advocacy Network. He is Past President of the Large Urology Group Practice Association. He is a founder for both CUSP Clinical Trials Consortium and DASHKO, a national urology practice data registry. He serves as the National Urology Research Director for 21st Century Oncology. He has served on the AUA Male Health Committee and the AUA Data Committee, the SITC Task Force for Prostate Cancer, the Bladder Cancer Advocacy Think Tank, and the Editorial Boards of Review in Urology, Urology Times, Chemotherapy Advisor, OncLive, PLOS ONE, Urology Practice, and World Journal of Urology. He serves as Editor of Everyday Urology-Oncology. Dr. Shore has written more than 200 peer-reviewed publications and numerous book chapters. He performs peer review for Lancet Oncology, New England Journal of Medicine, European Urology, the Journal of Urology, Urology, BJUI, PCPD, and numerous other high-impact scientific journals.
A graduate of Duke University and Duke University Medical School, Dr. Shore completed a 6-month clinical research fellowship in Pretoria, South Africa, and then completed his General Surgery/Urology training at New York Hospital Cornell Medical Center and at Memorial Sloan-Kettering Cancer Center in New York City. He is a Fellow of the American College of Surgeons.