Josh Mailman, MBA, presented “Neuroendocrine Tumors | Not so Rare?” during a joint session with SNMMI at the 2018 ASCO Annual Meeting.
How to cite: Mailman, Josh “Neuroendocrine Tumors | Not so Rare?” June 2, 2018. Accessed [date today]. https://dev.grandroundsinurology.com/neuroendocrine-tumors-not-so-rare/
Neuroendocrine Tumors | Not so Rare? – Summary:
Josh Mailman, MBA, the president of NorCal CarciNET, discusses the management of neuroendocrine tumors (NETs) through a patient’s perspective. He reports on overall patients’ attitudes toward Gallium Ga 68-DOTATATE imaging and the newly-approved Lutetium Lu 177 Dotatate therapy, as well as advocacy in the NETs patient community.
Evolution of NETs
Although it is the traditional assumption that NETs are rare, their incidence and prevalence have increased in recent years. These cancers are also currently the second most prevalent gastrointestinal (GI) cancer.
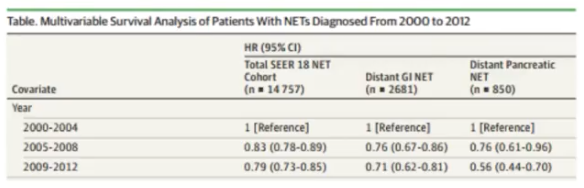
Fortunately, a multivariable survival analysis of patients diagnosed with NETs diagnosed from 2000 to 2012 shows an increase in overall survival (OS), as well.
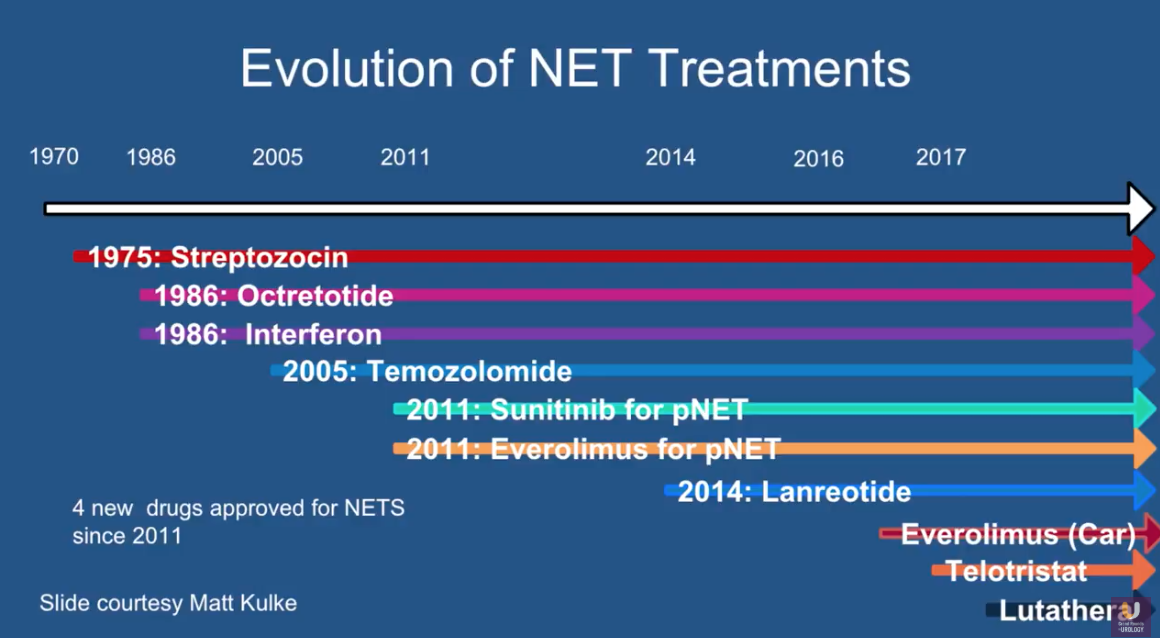
In 1975, the FDA approved the first treatment for NETs, streptozotocin. In the following years, there were not many other treatment options. However, there has been an influx of therapy advances in recent years. The FDA has approved four new drugs for NETs since 2011.
Finally, the most novel available treatment for NETs is Lutetium Lu 177 Dotatate (Lu 177, brand name Lutathera). This is a peptide receptor radionuclide therapy (PRRT) approved in January of this year.
A Patient’s Perspective on the NCCN Guidelines for NETs
Mailman is a NET patient who has undergone many rounds of PRRT with multiple different agents. Through this presentation, he offers his perspective of NETs management and recent advances. He first discusses patient opinions on the newly-released National Comprehensive Cancer Network (NCCN) guidelines for NETs.
He explains that the many options included in guidelines regarding the time of progression is a source of uncertainty and anxiety for patients. Patients seek care from different providers, like oncologists, nuclear medicine physicians, and other clinicians, each of who may recommend a different approach. Because of this, patients often receive many conflicting options they cannot evaluate themselves.
Gallium Ga 68-DOTATATE (Ga 68) Imaging
According to Mailman’s polls of other NET patients, as well as his own personal experience, the overall patient experience with Ga 68 is much better than other imaging methods. Unfortunately, although the FDA approved this agent in June of 2016, insurance companies are still claiming Ga 68 is experimental, therefore denying patients coverage. Advocacy groups, such as the Society of Nuclear Medicine and Molecular Imaging (SNMMI), are currently working to mitigate these coverage issues.
Lutathera/ PRRT
Although Lutathera has been available in Europe for many years, the FDA just approved this agent on January 26, 2018. Mailman polled the NET patient community about the effect of Lutathera’s approval on their care and their opinions and concerns about the therapy. He reports that overall, patients are glad to have access to the therapy in the United States. They have interest in participating in clinical trials to assess the safety of receiving more than four Luthathera treatments. However, the patient community has concerns regarding the therapy’s long- and short-term toxicity, insurance coverage and cost, complications with prior treatments, and the consequences of nuclear material in their bodies.
Mailman advises clinicians to explain to patients how Lutathera, or any new therapy, compares to their past treatments, when possible, to alleviate patient anxiety.
The NETs Patient Community
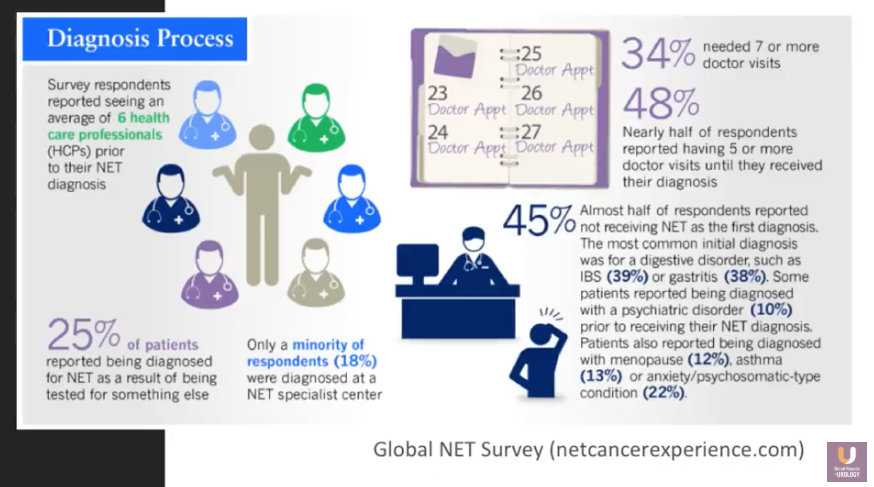
Mailman describes the challenges, unknown answers, and anxieties associated with the NET patient experience. He discusses the hardships of the diagnosis process, traveling to receive treatment and dealing with language barriers, clearing customs and other security checkpoints soon after receiving therapy, and evaluating the validity of information on the internet.
Furthermore, he explains ways in which engaged partnerships with patients and clinicians can lead to advances in NETs management and an improved patient experience. Patients can help recruit for clinical trials and advocate for pertinent legislation and FDA decisions.
ABOUT THE AUTHOR
Josh Mailman is President of the Northern California CarciNET Community. He is an internationally recognized advocate for neuroendocrine tumor patients as well as an advocate for integrative oncology, nuclear medicine, and molecular imaging. He is the inaugural chairman of the Society of Nuclear Medicine and Molecular Imaging’s (SNMMI) Patient Advocacy Advisory Board, a member of the Education and Research Foundation for Nuclear Medicine and Molecular Imaging (ERF) Board, and Treasurer of the Neuroendocrine Tumor Research Foundation. Mr. Mailman has an MBA from the Anderson School of Management at UCLA and has been a technology entrepreneur for more than 20 years.